Regulation of Dangerous Goods
Main ContentChapter 2.2 Class Specific Provisions
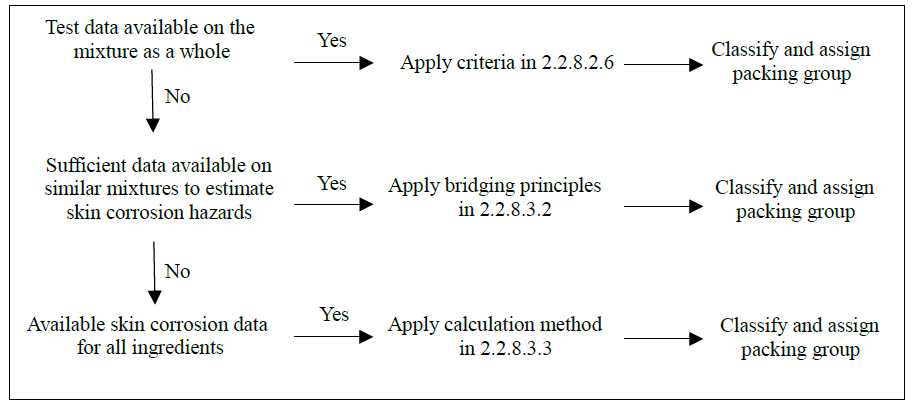
| 2.2.1 |
(Reserved) |
| 2.2.2 |
Class 2 – Gases |
|
|
|
| 2.2.2.1 |
Scope |
|
|
|
| 2.2.2.1.1 |
Class 2 includes gas substance which at 50°C has a vapour pressure greater than 300 kPa or is completely gaseous at 20°C at a standard pressure of 101.3 kPa. It comprises compressed gases, liquefied gases, dissolved gases, refrigerated liquefied gases, adsorbed gases, mixtures of one or more gases with one or more vapours of substances of other classes, articles charged with a gas, aerosols and chemicals under pressure. |
|
|
|
| 2.2.2.1.2 |
A gas is described according to its physical state as:
|
|
|
|
| 2.2.2.1.3 |
According to their chemical properties or physiological effects, gases may be flammable; non-flammable; non-toxic; toxic; supporters of combustion; corrosive; or may possess two or more of these properties simultaneously. |
|
|
|
| 2.2.2.2 |
Class subdivisions
Class 2 is subdivided according to the primary hazard of the gas: |
| 2.2.2.2.1 |
Class 2.1 Flammable gases
4 Flammability shall be determined by tests or calculation in accordance with methods adopted by the International Organization for Standardization (see ISO 10156:2017). Where insufficient data are available to use these methods, tests by a comparable method recognised by a national competent authority may be used. In this regard, it is necessary to provide the test report as the proof upon request. |
| 2.2.2.2.2 |
Class 2.2 Non-flammable, non-toxic gases
5 “Gases which may cause or contribute to the combustion of other material more than air does” means pure gases or gas mixtures with an oxidizing power greater than 23.5% as determined by a method specified in ISO 10156:2017. |
|
2.2.2.2.3 |
Class 2.3 Toxic gases6
Gases which: (a) are known to be so toxic or corrosive to humans as to pose a hazard to health; or (b) are presumed to be toxic or corrosive to humans because they have a LC50 value equal to or less than 5,000 ml/m3 (ppm).
6 Cases meeting the criteria 2.2.2.2.3 (a) or (b) owing to their corrosivity are to be classified as toxic with a subsidiary corrosive hazard. |
| 2.2.2.2.4 |
Gases and gas mixtures with hazards associated with more than one class take the following precedence:
(a) Class 2.3 takes precedence over all other classes; (b) Class 2.1 takes precedence over Class 2.2. |
| 2.2.2.2.5 |
Pursuant to Cap. 295E, the following Class 2 DG are not subject to Dangerous Goods Ordinance:
|
| 2.2.2.2.6 |
As the list in 2.2.2.2.5 is not exhaustive, further reference shall be made to Cap. 295E for clarifications. |
| 2.2.2.3 |
Mixtures of gases
(a) Flammability shall be determined by tests or calculation in accordance with methods adopted by the International Organization for Standardization (see ISO Standard 10156:2017). Where insufficient data are available to use these methods, tests by a comparable method recognised by a national competent authority may be used. In this regard, it is necessary to provide the test report as the proof upon request; and |
| 2.2.2.3.1 |
A gas mixture has a subsidiary hazard of corrosivity when the mixture is known by human experience to be destructive to the skin, eyes or mucous membranes or when the LC50 value of the corrosive components of the mixture is equal to or less than 5,000 ml/m3 (ppm) by calculation in 2.2.3.2 in IMDG Code or 2.2.3(c) in Recommendations on the Transport of Dangerous Goods Model Regulations. |
| 2.2.2.3.2 |
Oxidizing ability is determined either by tests or by calculation methods adopted by ISO (see the Note in 2.2.2.2.2(b) and ISO 10156:2017). |
| 2.2.3 |
Class 3 – Flammable Liquids |
|
|
|
| 2.2.3.1 |
Scope |
|
|
|
| 2.2.3.1.1 |
Class 3 includes flammable liquids and liquid desensitized explosives. Flammable liquid items/liquid desensitized explosives having specific UN numbers shall be classified as stated in the DG List, including the packing groups, subsidiary hazards etc. For the items which are not specified in DG List but possible to be classified as Class 3 DG, the flashpoint, initial boiling point and viscosity shall be determined. In general, flammable liquids are liquids, or mixture of liquids, or liquids containing solids in solution or suspension (such as paints, varnishes, lacquers, etc., but not including substances which, on account of their other dangerous characteristics, have been included in other classes) which have a flashpoint at or below 60°C (closed-cup test). In countries where it is customary to determine flashpoints by the open-cup method, the temperatures given by that method would need to be reduced to correspond with those in the Code. |
|
|
|
| 2.2.3.1.2 |
The flashpoint of a flammable liquid is the lowest temperature of the liquid at which its vapour forms an ignitable mixture with air. It gives a measure of the risk of formation of explosive or ignitable mixtures when the liquid escapes from its packing. A flammable liquid cannot be ignited so long as its temperature remains below the flashpoint. |
|
|
|
| 2.2.3.1.3 |
The test methods of flashpoint determination can be divided into two groups, depending on the use in an apparatus of an open receptacle (open-cup methods) or a closed one which is only opened to admit the flame (closed-cup methods). As a rule, the flashpoints found in an open-cup test are a few degrees higher than in a closed-cup test. In general, reproducibility in closed-cup apparatus is better than in open-cup. It is therefore flashpoints shall be determined by means of closed-cup methods. |
|
|
|
| 2.2.3.1.4 |
Liquid desensitized explosives are explosive substances (UN 1204, UN 2059, UN 3064, UN 3343, UN 3357 and UN 3379 in the DG List) which are dissolved or suspended in water or other liquid substances, to form a homogeneous liquid mixture to suppress their explosive properties. |
|
|
|
| 2.2.3.1.5 |
Liquids meeting the definition in 2.2.3.1.1 with a flashpoint of more than 35°C which do not sustain combustion need not be considered as flammable liquid. Liquids are considered to be unable to sustain combustion for the purposes of the Code if:
(a) they have passed the suitable combustibility test7; or (b) their fire point according to ISO 2592:2017 is greater than 100°C; or (c) they are water-miscible solutions with a water content of more than 90%, by mass.
7 The Sustained Combustibility Test prescribed in Part III, sub-section 32.5.2 of the United Nations Manual of Tests and Criteria. |
|
|
|
|
2.2.3.2 |
Assignment of packing group of Class 3 DG |
| 2.2.3.2.1 |
Flammable liquids are grouped for packing purposes according to their flashpoint, their initial boiling point, and their viscosity8. Table in 2.2.3.2.2 shows the packing group for the substance whose only hazard is flammability.
8 Reference method for viscosity determination may be found in Part III, sub-section 32.4.3 of the United Nations Manual of Tests and Criteria. Where the substance concerned is non-Newtonian, or when a flow cup method of viscosity determination is unsuitable, a variable shear-rate viscometer shall be used to determine the dynamic viscosity coefficient of the substance, at 23°C, at a number of shear-rates. The values obtained are plotted against shear rate and then extrapolated to zero shear rate. The dynamic viscosity thus obtained, divided by the density, gives the apparent kinematic viscosity at near-zero shear rate. |
||||||||||||||||||||||||||||
| 2.2.3.2.2 |
Packing group based on flammability
|
||||||||||||||||||||||||||||
| 2.2.3.2.3 | For a liquid with additional hazard(s), the packing group determined from 2.2.3.2.2 and the packing group based on the severity of the additional hazard(s) shall be considered, and the classification and packing group determined in accordance with the provisions in Chapter 2.1. | ||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
| 2.2.3.2.4 |
Viscous flammable liquids such as paints, enamels, lacquers, varnishes, adhesives and polishes with a flashpoint of less than 23°C may be placed in packing group III in conformity with the procedures prescribed in the United Nations Manual of Tests and Criteria, Part III sub-section 32.3, which provided that:
(b) the mixture or any separated solvent does not meet the criteria for Class 6.1 or Class 8; (c) the capacity of the receptacle used does not exceed 450 L; and (d) the viscosity and flashpoint are in accordance with the following table:
|
||||||||||||||||||||||||||||
| 2.2.3.2.5 |
The flashpoint of a flammable liquid may be altered by the presence of an impurity. The substances listed in Class 3 DG in the DG List shall generally be regarded as chemically pure. Since commercial products may contain added substances or impurities, flashpoints may vary, and this may have an effect on classification or determination of the packing group for the product. In the event of doubt regarding the classification or packing group of a substance, the flashpoint of the substance shall be determined experimentally. The reference methods for determining the flashpoint of flammable liquids may be found in 2.3.3.6 in IMDG Code or 2.3.3 in Recommendations on the Transport of Dangerous Goods Model Regulations. |
||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
| 2.2.3.2.6 |
The reference methods for determining the initial boiling point of flammable liquids may be found in 2.3.4 in IMDG Code or 2.3.4 in Recommendations on the Transport of Dangerous Goods Model Regulations. |
| 2.2.3a |
Class 3A – Diesel or Fuel Oil or Furnace Oil |
|
|
|
| 2.2.3a.1 |
Scope |
|
|
|
| 2.2.3a.1.1 |
For the purposes of the Ordinance, Class 3A DG include diesel oils (distillates and/or light residuals), furnace oils and other fuel oils having a flashpoint exceeding 60℃ (closed-cup test). Class 3A DG are assigned with HK number H301 and listed in Part 4 of Schedule 2 of Cap. 295E. |
|
|
| HK No. | Proper Shipping Name | Packing Group | |
| H301 | DIESEL or FUEL OIL or FURNACE OIL | III |
| 2.2.4 |
Class 4 – Flammable Solids; Substances Liable to Spontaneous Combustion; Substances which, in Contact with Water, Emit Flammable Gases |
|
|
|
| 2.2.4.1 |
Scope |
|
|
|
| 2.2.4.1.1 |
Class 4 DG are subdivided as follows:
(a) Class 4.1 – Flammable solids Solids which are readily combustible or may cause or contribute to fire through friction; self-reactive substances (solids and liquids) and polymerizing substances which are liable to undergo a strong exothermic reaction; solid desensitized explosives which may explode if not diluted sufficiently;
(b) Class 4.2 – Substances liable to spontaneous combustion Substances (solids and liquids) which are liable to spontaneous heating, or to heating up in contact with air, and being then liable to catch fire;
(c) Class 4.3 – Substances which, in contact with water, emit flammable gases Substances (solids and liquids) which, by interaction with water, are liable to become spontaneously flammable or to give off flammable gases in dangerous quantities. |
|
|
|
| 2.2.4.1.2 |
Class 4 DG having specific UN numbers shall be classified as stated in the DG List, including the packing groups, subsidiary hazards etc. However, for following substances which are not specified in the DG List but have properties possible to be classified as Class 4 DG, the corresponding test methods and criteria including advice on application of the tests are given in the United Nations Manual of Tests and Criteria for the classification9 :
(a) flammable solids (Class 4.1); (b) self-reactive substances (Class 4.1); (c) polymerizing substances (Class 4.1); (d) pyrophoric solids (Class 4.2); (e) pyrophoric liquids (Class 4.2); (f) self-heating substances (Class 4.2); and (g) substances which, in contact with water, emit flammable gases (Class 4.3).
9 Test methods and criteria for self-reactive substances and polymerizing substances are given in Part II of the United Nations Manual of Tests and Criteria, and test methods and criteria for the other types of substances of Class 4 DG are given in the United Nations Manual of Tests and Criteria, Part III, section 33. |
| 2.2.4.1.3 |
Class 4.1 DG include the following:
(a) flammable solids; (b) self-reactive substances; (c) solid desensitized explosives; and (d) polymerizing substances. |
|
|
|
| 2.2.4.1.4 |
Flammable solids under Class 4.1 |
|
|
|
| 2.2.4.1.4.1 |
Flammable solids mean readily combustible solids and solids which may cause fire through friction. |
|
|
|
| 2.2.4.1.4.2 |
Readily combustible solids mean fibres, powdered, granular, or pasty substances which are dangerous if they can be easily ignited by brief contact with an ignition source, and if the flame spreads rapidly. The danger may come not only from the fire but also from toxic combustion products. Metal powders are especially dangerous because of the difficulty of extinguishing a fire, since normal extinguishing agents such as carbon dioxide or water can increase the hazard. |
|
|
|
| 2.2.4.1.4.3 |
Powdered, granular or pasty substances shall be classified as readily combustible solids of Class 4.1 when the time of burning of one or more of the test runs, performed in accordance with the test method described in the United Nations Manual of Tests and Criteria, Part III, sub-section 33.2.1, is less than 45 s or the rate of burning is more than 2.2 mm/s. Powders of metals or metal alloys shall be classified as Class 4.1 when they can be ignited and the reaction spreads over the whole length of the sample in 10 minutes or less.
|
| 2.2.4.1.4.4 |
Solids which may cause fire through friction shall be classified as Class 4.1 by analogy with existing entries until definitive criteria are established. |
|
|
|
| 2.2.4.1.4.5 |
Assignment of packing groups of flammable solids |
|
|
|
| 2.2.4.1.4.5.1 |
Criteria for the packing group assignment of Class 4.1 flammable solids are specified in the test method described in the United Nations Manual of Tests and Criteria, Part III, sub-section 33.2.1. For readily combustible solids (other than metal powders), packing group II shall be assigned if the burning time is less than 45 s and the flame passes the wetted zone. Packing group II shall be assigned to powders of metal or metal alloys if the zone of reaction spreads over the whole length of the sample in five minutes or less. |
|
|
|
| 2.2.4.1.4.5.2 |
For readily combustible solids (other than metal powders), packing group III shall be assigned if the burning time is less than 45 s and the wetted zone stops the flame propagation for at least four minutes. Packing group III shall be assigned to metal powders if the reaction spreads over the whole length of the sample in more than five minutes but not more than ten minutes. |
|
|
|
| 2.2.4.1.4.5.3 |
For solids which may cause fire through friction, the packing group shall be assigned by analogy with existing entries or in accordance with any appropriate special provision. |
|
|
|
| 2.2.4.1.4.5.4 |
Pyrophoric metal powders, if wetted with sufficient water to suppress their pyrophoric properties, may be classified as Class 4.1. |
|
|
|
| 2.2.4.1.5 |
Self-reactive substances under Class 4.1 |
|
|
|
| 2.2.4.1.5.1 |
Self-reactive substances10 are thermally unstable substances liable to undergo a strong exothermic decomposition even without participation of oxygen (air). Substances are not considered to be self-reactive substances of Class 4.1, if:
(a) they are Class 1 explosives; (b) they are Class 5.1 oxidizing substances except that mixtures of oxidizing substances which contain 5.0% or more of combustible organic substances11 ; (c) they are Class 5.2 organic peroxides; (d) their heat of decomposition12 is less than 300 J/g; or (e) their self-accelerating decomposition temperature (SADT) (see 2.2.4.1.5.11) is greater than 75°C for a 50 kg package.
10 Any substance which shows the properties of a self-reactive substance shall be classified as such, even if this substance gives a positive test result according to 2.2.4.2.3 – 2.2.4.2.7 for inclusion in Class 4.2. |
|
|
|
| 2.2.4.1.5.2 |
A mixture showing the properties of a self-reactive substance, type B to F, shall be classified as a self-reactive substance of Class 4.1. |
|
|
|
| 2.2.4.1.5.3 |
A mixture showing the properties of a self-reactive substance, type G, but not listed in 2.2.4.5 shall be considered for classification as a substance of Class 5.1 (see 2.2.5.2). |
|
|
|
| 2.2.4.1.5.4 |
The decomposition of self-reactive substances can be initiated by heat, contact with catalytic impurities (such as acids, heavy-metal compounds, bases), friction or impact. The rate of decomposition increases with temperature and varies with the substance. Decomposition, particularly if no ignition occurs, may result in the evolution of toxic gases or vapours. For certain self-reactive substances, the temperature shall be controlled. Some self-reactive substances may decompose explosively, particularly if confined. This characteristic may be modified by the addition of diluents or by the use of appropriate packagings. Some self-reactive substances burn vigorously. Self-reactive substances are, for example, some compounds of the types listed below:
(a) aliphatic azo compounds (–C–N=N–C–); (b) organic azides (–C–N3); (c) diazonium salts (–CN2+Z-); (d) N-nitroso compounds (–N–N=O); and (e) aromatic sulphonylhydrazides (–SO2–NH–NH2).
This list is not exhaustive and substances with other reactive groups and some mixtures of substances may have similar properties. |
|
|
|
| 2.2.4.1.5.5 |
Self-reactive substances are classified into seven types according to the degree of danger they present. The types of self-reactive substance range from type A, which is prohibited goods, to type G, which is not subject to the provisions for self-reactive substances of Class 4.1. The classification of types B to F is directly related to the maximum quantity allowed in one packaging. |
|
|
|
| 2.2.4.1.5.6 |
Permitted self-reactive substances are listed in 2.2.4.5. For each permitted substance listed, the appropriate generic entry of the DG List (UN 3221 to UN 3240) is assigned, and appropriate subsidiary risks and remarks are given.13 In 2.2.4.5, the permitted substances are classified as technically pure substances (except where the concentration of less than 100% is specified).
13 Generic entries specify self-reactive substance type (B to F); physical state (liquid or solid); and temperature control, when required. |
|
|
|
| 2.2.4.1.5.7 |
For other concentrations, the substances may be classified differently. Classification of these self-reactive substances shall be made by the competent authority of the country of origin on the basis of a test report. The applicable classification procedures, test methods and criteria, and an example of a suitable test report, are given in the United Nations Manual of Tests and Criteria, Part II. The statement of approval shall contain the classification and other relevant conditions. Such statement of approval and the corresponding test report shall be provided as proof upon request. |
|
|
|
| 2.2.4.1.5.8 |
Activators, such as zinc compounds, may be added to some self-reactive substances to change their reactivity. Depending on both the type and the concentration of the activator, this may result in a decrease in thermal stability and a change in explosive properties. If the thermal stability or explosive properties of self-reactive substances are altered by adding activators, the new formulation shall be assessed in accordance with this classification procedure. |
|
|
|
| 2.2.4.1.5.9 |
Samples of self-reactive substances or formulations of self-reactive substances not listed in 2.2.4.5, for which a complete set of test results is not available and which is required for further testing or evaluation, may be assigned to one of the appropriate entries for self-reactive substances type C provided the following conditions are met:
(a) the available data indicate that the sample would be no more dangerous than self-reactive substances type B; (b) the sample is appropriately packaged and the quantity is limited to 10 kg; and (c) the available data indicate that the control temperature, if any, is sufficiently low to prevent any dangerous decomposition and sufficiently high to prevent any dangerous phase separation. |
|
|
|
| 2.2.4.1.5.10 |
A self-reactive substance is regarded as possessing explosive properties when, in laboratory testing, the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement. These properties shall be determined experimentally14.
14 Suitable test methods with pertinent evaluation criteria are given in the United Nations Manual of Tests and Criteria, Part II. |
|
|
|
| 2.2.4.1.5.11 |
Self-reactive substances are subject to temperature control if their SADT is less than or equal to 55°C. Test methods for determining the SADT are given in the Manual of Tests and Criteria, Part II, Chapter 28. For currently assigned self-reactive substances, the control and emergency temperatures are shown in 2.2.4.5. Self-reactive substances requiring temperature control shall refer to 7.3.7.2 in IMDG Code or 7.1.5.3 in Recommendations on the Transport of Dangerous Goods Model Regulations. |
|
|
|
| 2.2.4.1.5.12 |
Self-reactive substances may be desensitized through the use of a diluent. If a diluent is used, the self-reactive substance shall be tested with the diluent present in the concentration and form used. |
|
|
|
| 2.2.4.1.5.13 |
Diluents which may allow a self-reactive substance to concentrate to a dangerous extent in the event of leakage from a package shall not be used. |
|
|
|
| 2.2.4.1.5.14 |
The diluent shall be compatible with the self-reactive substance. In this regard, compatible diluents are those solids or liquids which have no detrimental influence on the thermal stability and hazard type of the self-reactive substance. |
|
|
|
| 2.2.4.1.5.15 |
Liquid diluents in liquid formulations requiring temperature control shall have a boiling point of at least 60°C and a flashpoint not less than 5°C. The boiling point of the liquid shall be at least 50°C higher than the control temperature of the self-reactive substance. |
|
|
|
| 2.2.4.1.6 |
Solid desensitized explosives under Class 4.1 |
|
|
|
| 2.2.4.1.6.1 |
Solid desensitized explosives are explosive substances which are wetted with water or alcohols or are diluted with other substances to form a homogeneous solid mixture to suppress their explosive properties. Suitable and compatible solvent, such as alcohol, may have to be added to lower the freezing point of the liquid. Some of these substances, when in a dry state, are classified as explosives. Where reference is made to a substance which is wetted with water, or some other liquid, it shall be assigned as a Class 4.1 substance only when in the wetted condition specified. Entries in the DG List for solid desensitized explosives are UN 1310, UN 1320, UN 1321, UN 1322, UN 1336, UN 1337, UN 1344, UN 1347, UN 1348, UN 1349, UN 1354, UN 1355, UN 1356, UN 1357, UN 1517, UN 1571, UN 2555, UN 2556, UN 2557, UN 2852, UN 2907, UN 3317, UN 3319, UN 3344, UN 3364, UN 3365, UN 3366, UN 3367, UN 3368, UN 3369, UN 3370, UN 3376, UN 3380 and UN 3474. |
|
|
|
| 2.2.4.1.6.2 |
Substances that:
(a) have been provisionally accepted into Class 1 according to Test Series 1 and 2 but exempted from Class 1 by Test Series 6; (b) are not self-reactive substances of Class 4.1; and (c) are not substances of Class 5;
are also assigned to Class 4.1. UN 2956, UN 3241, UN 3242 and UN 3251 are such entries. |
|
|
|
| 2.2.4.1.7 |
Polymerzing substances and mixture (stabilized) under Class 4.1 |
|
|
|
| 2.2.4.1.7.1 |
Polymerizing substances are substances which, without stabilization, are liable to undergo a strong exothermic reaction resulting in the formation of larger molecules or resulting the formation of polymers under conditions normally encountered. Such substances are considered to be polymerizing substances of Class 4.1 when:
(a) their self-accelerating polymerization temperature (SAPT) is 75°C or less under the conditions (with or without chemical stabilization) and in appropriate packaging; (b) they exhibit a heat of reaction of more than 300 J/g; and (c) they do not meet any other criteria for inclusion in Classes 1 to 8. |
|
|
|
| 2.2.4.1.7.2 |
A mixture meeting the criteria of a polymerizing substance shall be classified as a polymerizing substance of Class 4.1. |
|
|
|
| 2.2.4.1.7.3 |
Polymerizing substances requiring temperature control shall refer to 7.3.7.2 in IMDG Code or 7.1.5.3 in Recommendations on the Transport of Dangerous Goods Model Regulations. |
| 2.2.4.2 |
Class 4.2 – Substances liable to spontaneous combustion |
|
|
|
| 2.2.4.2.1 |
Class 4.2 DG comprises:
(a) Pyrophoric substances, which are substances, including mixtures and solutions (liquid or solid), which, even in small quantities, ignite within 5 minutes of coming into contact with air. These substances are the most liable to spontaneous combustion; and (b) Self-heating substances, which are substances, other than pyrophoric substances, which, in contact with air without energy supply, are liable to self-heating. These substances will ignite only when in large amounts (kilograms) and after long periods of time (hours or days). |
|
|
|
| 2.2.4.2.2 |
Self-heating of a substance is a process where the gradual reaction of that substance with oxygen (in air) generates heat. If the rate of heat production exceeds the rate of heat loss, then the temperature of the substance will rise which, after an induction time, may lead to self-ignition and combustion. |
|
|
|
| 2.2.4.2.3 |
Solids are considered pyrophoric solids which shall be classified in Class 4.2 if, in tests performed in accordance with the test method given in the United Nations Manual of Tests and Criteria, Part III, Section 33 Test N.2, the sample ignites in one of the tests. |
|
|
|
| 2.2.4.2.4 |
Liquids are considered pyrophoric liquids which shall be classified in Class 4.2 if, in tests performed in accordance with the test method given in the United Nations Manual of Tests and Criteria, Part III, Section 33 Test N.3, the liquid ignites in the first part of the test, or if it ignites or chars the filter paper. |
|
|
|
| 2.2.4.2.5 |
A substance shall be classified as a self-heating substance of Class 4.2 if, in tests performed in accordance with the test method given in the United Nations Manual of Tests and Criteria, Part III, Section 33 Test N.4:
(a) a positive result is obtained using a 25 mm cube sample at 140°C; (b) a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 100 mm cube sample at 120°C and the substance is in packages with a volume of more than 3 m3; (c) a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 100 mm cube sample at 100°C and the substance is in packages with a volume of more than 450 L; (d) a positive result is obtained in a test using a 100 mm cube sample at 140°C and a positive result is obtained using a 100 mm cube sample at 100°C. |
|
|
|
| 2.2.4.2.6 |
Self-reactive substances, giving also a positive result with this test method shall not be classified in Class 4.2 but in Class 4.1 (see 2.2.4.1.5). |
|
|
|
| 2.2.4.2.7 |
A substance shall not be classified in Class 4.2 if:
(a) a negative result is obtained in a test using a 100 mm cube sample at 140°C; (b) a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 25 mm cube sample at 140°C, a negative result is obtained in a test using a 100 mm cube sample at 120°C and the substance is in packages with a volume not more than 3 m3; (c) a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 25 mm cube sample at 140°C, a negative result is obtained in a test using a 100 mm cube sample at 100°C and the substance is in packages with a volume not more than 450 L. |
| 2.2.4.3 | Class 4.3 – Substances which, in contact with water, emit flammable gases |
| 2.2.4.3.1 | Class 4.3 substances are either liquids or solids which, by interaction with water, are liable to become spontaneously flammable or to give off flammable gases in dangerous quantities. |
| 2.2.4.3.2 | Certain substances, in contact with water, may emit flammable gases that can form explosive mixtures with air. Such mixtures are easily ignited by all ordinary sources of ignition and the resulting blast wave and flames may endanger people and the environment. |
| 2.2.4.3.3 |
Substances which, in contact with water, emit flammable gases shall be classified in Class 4.3 if, in tests performed in accordance with the test method given in the United Nations Manual of Tests and Criteria, Part III, Section 33 Test N.5 15:
(a) spontaneous ignition takes place in any step of the test procedure; or (b) evolution of flammable gas at a rate greater than 1 litre per kilogram of the substance per hour.
15 This test method shall not be applied to pyrophoric substances. |
|
|
|
| 2.2.4.3.4 |
Assignment of packing groups of substances which, in contact with water, emit flammable gases |
|
|
| Packing Group | Criteria | |
| I |
(i) any substance which reacts vigorously with water at ambient temperatures and demonstrates generally a tendency for the gas produced to ignite spontaneously, or (ii) reacts readily with water at ambient temperatures such that the rate of evolution of flammable gas ≥ 10 litres per kilogram of substances over any one minute. |
|
| II |
(i) any substance which reacts readily with water at ambient temperatures such that the maximum rate of evolution of flammable gas ≥ 20 litres per kilogram of substances per hour, and (ii) does not meet the criteria for packing group I. |
|
| III |
(i) any substance which reacts slowly with water at ambient temperatures such that the maximum rate of evolution of flammable gas >1 litre per kilogram of substances per hour, and (ii) does not meet the criteria for packing group I or II. |
| 2.2.4.4 | Organometallic Substances |
| 2.2.4.4.1 | Depending on their properties, organometallic substances can be classified in Class 4.2 or Class 4.3 (UN 3391 to UN 3400) with additional subsidiary hazards. The required tests methods (N1 to N5) can be found in the Manual of Tests and Criteria, Part III, Section 33. |
| 2.2.4.5 |
List of currently assigned self-reactive substances
Note 1: The classification given in this table is based on the technically pure substance (except where a concentration of less than 100% is specified). For other concentrations, the substances may be classified differently following the procedures in 2.2.4.1.5.10 to 2.2.4.1.5.11.
Note 2: In the column “Packing Method”, codes “OP1” to “OP8” refer to packing methods in basic packing instruction BP520. |
|
SELF-REACTIVE SUBSTANCE |
Concentration (%) |
Packing method (BP520) |
Control temperature (°C) |
Emergency temperature (°C) |
UN |
Remarks |
|
ACETONE-PYROGALLOL COPOLYMER 2- DIAZO-1-NAPHTHOL-5-SULPHONATE |
100 |
OP8 |
|
|
3228 |
|
|
AZODICARBONAMIDE FORMULATION TYPE B, TEMPERATURE CONTROLLED |
< 100 |
OP5 |
|
|
3232 |
(1) |
|
AZODICARBONAMIDE FORMULATION TYPE C |
< 100 |
OP6 |
|
|
3224 |
(3) |
|
AZODICARBONAMIDE FORMULATION TYPE C, TEMPERATURE CONTROLLED |
< 100 |
OP6 |
|
|
3234 |
(4) |
|
AZODICARBONAMIDE FORMULATION TYPE D |
< 100 |
OP7 |
|
|
3226 |
(5) |
|
AZODICARBONAMIDE FORMULATION TYPE D, TEMPERATURE CONTROLLED |
< 100 |
OP7 |
|
|
3236 |
(6) |
|
2,2' -AZODI(2,4-DIMETHYL-4-METHOXYVALERONITRILE) |
100 |
OP7 |
-5 |
+5 |
3236 |
|
|
2,2' -AZODI(2,4-DIMETHYLVALERONITRILE) |
100 |
OP7 |
+10 |
+15 |
3236 |
|
|
2,2' -AZODI(ETHYL-2-METHYLPROPIONATE) |
100 |
OP7 |
+20 |
+25 |
3235 |
|
|
1,1' -AZODI(HEXAHYDROBENZONITRILE) |
100 |
OP7 |
|
|
3226 |
|
|
2,2'-AZODI(ISOBUTYRONITRILE) |
100 |
OP6 |
+40 |
+45 |
3234 |
|
|
2,2'-AZODI(ISOBUTYRONITRILE) as a water-based paste |
≤ 50 |
OP6 |
|
|
3224 |
|
|
2,2'-AZODI(2-METHYLBUTYRONITRILE) |
100 |
OP7 |
+35 |
+40 |
3236 |
|
|
BENZENE-1,3-DISULPHONYL HYDRAZIDE, as a paste |
52 |
OP7 |
|
|
3226 |
|
|
BENZENESULPHONYL HYDRAZIDE |
100 |
OP7 |
|
|
3226 |
|
|
4-(BENZYL(ETHYL)AMINO)-3-ETHOXY- BENZENEDIAZONIUM ZINC CHLORIDE |
100 |
OP7 |
|
|
3226 |
|
|
4-(BENZYL(METHYL)AMINO)-3-ETHOXYBENZENEDIAZONIUM ZINC CHLORIDE |
100 |
OP7 |
+40 |
+45 |
3236 |
|
|
3-CHLORO-4-DIETHYLAMINOBENZENEDIAZONIUM ZINC CHLORIDE |
100 |
OP7 |
|
|
3226 |
|
|
2-DIAZO-1-NAPHTHOL-4-SULPHONYL CHLORIDE |
100 |
OP5 |
|
|
3222 |
|
|
2-DIAZO-1-NAPHTHOL-5-SULPHONYL CHLORIDE |
100 |
OP5 |
|
|
3222 |
|
|
2-DIAZO-1-NAPHTHOLSULPHONIC ACID ESTER MIXTURE TYPE D |
<100 |
OP7 |
|
|
3226 |
(9) |
|
2,5-DIBUTOXY-4-(4-MORPHOLINYL)BENZENEDIAZONIUM TETRACHLOROZINCATE (2:1) |
100 |
OP8 |
|
|
3228 |
|
|
2,5-DIETHOXY-4-MORPHOLINO- BENZENEDIAZONIUM ZINC CHLORIDE |
67-100 |
OP7 |
+35 |
+40 |
3236 |
|
|
2,5-DIETHOXY-4-MORPHOLINO- BENZENEDIAZONIUM ZINC CHLORIDE |
66 |
OP7 |
+40 |
+45 |
3236 |
|
|
2,5-DIETHOXY-4-MORPHOLINO- BENZENEDIAZONIUM TETRAFLUOROBORATE |
100 |
OP7 |
+30 |
+35 |
3236 |
|
|
2,5-DIETHOXY-4-(4-MORPHOLINYL)- BENZENEDIAZONIUM SULPHATE |
100 |
OP7 |
|
|
3226 |
|
|
2,5-DIETHOXY-4-(PHENYLSULPHONYL)-BENZENEDIAZONIUM ZINC CHLORIDE |
67 |
OP7 |
+40 |
+45 |
3236 |
|
|
DIETHYLENEGLYCOL BIS(ALLYLCARBONATE) + DI-ISOPROPYL PEROXYDICARBONATE |
≥ 88 + ≤ 12 |
OP8 |
-10 |
0 |
3237 |
|
|
2,5-DIMETHOXY-4-(4-METHYLPHENYL-SULPHONYL)BENZENEDIAZONIUM ZINC CHLORIDE |
79 |
OP7 |
+40 |
+45 |
3236 |
|
|
4-(DIMETHYLAMINO)-BENZENEDIAZONIUM TRICHLOROZINCATE(-1) |
100 |
OP8 |
|
|
3228 |
|
|
4-DIMETHYLAMINO-6-(2-DIMETHYLAMINOETHOXY)TOLUENE-2-DIAZONIUM ZINC CHLORIDE |
100 |
OP7 |
+40 |
+45 |
3236 |
|
|
N,N'-DINITROSO-N,N'-DIMETHYL TEREPHTHALAMIDE, as a paste |
72 |
OP6 |
|
|
3224 |
|
|
N,N'-DINITROSOPENTAMETHYLENE- TETRAMINE |
82 |
OP6 |
|
|
3224 |
(7) |
|
DIPHENYLOXIDE-4,4'-DISULPHONYL HYDRAZIDE |
100 |
OP7 |
|
|
3226 |
|
|
4-DIPROPYLAMINOBENZENE- DIAZONIUM ZINC CHLORIDE |
100 |
OP7 |
|
|
3226 |
|
|
2-(N,N-ETHOXYCARBONYL- PHENYLAMINO)-3-METHOXY-4-(N-METHYL-N-CYCLOHEXYLAMINO)BENZENE-DIAZONIM ZINC CHLORIDE |
63-92 |
OP7 |
+40 |
+45 |
3236 |
|
|
2-(N,N-ETHOXYCARBONYL- PHENYLAMINO)-3-METHOXY-4-(N-METHYL-N-CYCLOHEXYLAMINO)BENZENE-DIAZONIUM ZINC CHLORIDE |
62 |
OP7 |
+35 |
+40 |
3236 |
|
|
N-FORMYL-2-(NITROMETHYLENE)-1,3-PERHYDROTHIAZINE |
100 |
OP7 |
+45 |
+50 |
3236 |
|
|
2-(2-HYDROXYETHOXY)-1- (PYRROLIDIN-1-YL)BENZENE-4- DIAZONIUM ZINC CHLORIDE |
100 |
OP7 |
+45 |
+50 |
3236 |
|
|
3-(2-HYDROXYETHOXY)-4- (PYRROLIDIN-1-YL)BENZENE-DIAZONIUM ZINC CHLORIDE |
100 |
OP7 |
+40 |
+45 |
3236 |
|
|
2-(N,N-METHYLAMINOETHYL-CARBONYL)-4-(3,4-DIMETHYL-PHENYLSULPHONYL)BENZENE- DIAZONIUM HYDROGEN SULPHATE |
96 |
OP7 |
+45 |
+50 |
3236 |
|
|
4-METHYLBENZENESULPHONYL- HYDRAZIDE |
100 |
OP7 |
|
|
3226 |
|
|
3-METHYL-4-(PYRROLIDIN-1-YL) BENZENEDIAZONIUM TETRAFLUOROBORATE |
95 |
OP6 |
+45 |
+50 |
3234 |
|
|
4-NITROSOPHENOL |
100 |
OP7 |
+35 |
+40 |
3236 |
|
|
PHOSPHOROTHIOIC ACID, O- [(CYANOPHENYLMETHYLENE)- AZANYL]-O,O-DIETHYL ESTER |
82-91 (Z isomer) |
OP8 |
|
|
3227 |
(10) |
|
SELF-REACTIVE LIQUID, SAMPLE |
|
OP2 |
|
|
3223 |
(8) |
|
SELF-REACTIVE LIQUID, SAMPLE, TEMPERATURE CONTROLLED |
|
OP2 |
|
|
3233 |
(8) |
|
SELF-REACTIVE SOLID, SAMPLE |
|
OP2 |
|
|
3224 |
(8) |
|
SELF-REACTIVE SOLID, SAMPLE, TEMPERATURE CONTROLLED |
|
OP2 |
|
|
3234 |
(8) |
|
SODIUM 2-DIAZO-1-NAPHTHOL-4-SULPHONATE |
100 |
OP7 |
|
|
3226 |
|
|
SODIUM 2-DIAZO-1-NAPHTHOL-5-SULPHONATE |
100 |
OP7 |
|
|
3226 |
|
|
TETRAMINEPALLADIUM(II) NITRATE |
100 |
OP6 |
+30 |
+35 |
3234 |
|
|
Remarks:
(1) Azodicarbonamide formulations which fulfill the criteria of 20.4.2(b) of the Manual of Tests and Criteria. The control and emergency temperatures depend on their SADT determined by the Manual of Tests and Criteria, Part II, Section 20 and Section 28.4 and if required, it shall refer to the control requirements in 7.3.7.2 in IMDG Code or 7.1.5.3 in Recommendations on the Transport of Dangerous Goods Model Regulations. (2) (reserved) (3) Azodicarbonamide formulations which fulfil the criteria of 20.4.2(c) of the Manual of Tests and Criteria. (4) Azodicarbonamide formulations which fulfil the criteria of 20.4.2(c) of the Manual of Tests and Criteria. The control and emergency temperatures depend on their SADT determined by the Manual of Tests and Criteria, Part II, Section 20 and Section 28.4 and if required, it shall refer to the control requirements in 7.3.7.2 in IMDG Code or 7.1.5.3 in Recommendations on the Transport of Dangerous Goods Model Regulations. (5) Azodicarbonamide formulations which fulfil the criteria of 20.4.2(d) of the Manual of Tests and Criteria. (6) Azodicarbonamide formulations which fulfil the criteria of 20.4.2(d) of the Manual of Tests and Criteria. The control and emergency temperatures depend on their SADT determined by the Manual of Tests and Criteria, Part II, Section 20 and Section 28.4 and if required, it shall refer to the control requirements in 7.3.7.2 in IMDG Code or 7.1.5.3 in Recommendations on the Transport of Dangerous Goods Model Regulations. (7) With compatible diluent having a boiling point of not less than 150°C. (8) See 2.2.4.1.5.9. (9) This entry applies to mixtures of esters of 2-diazo-1-naphthol-4-sulphonic acid and 2-diazo-1-naphthol-5-sulphonic acid meeting the criteria of 20.4.2(d) of the Manual of Tests and Criteria. (10) This entry applies to the technical mixture in n-butanol within the specified concentration limits of the (Z) isomer. |
| 2.2.5 |
Class 5 - Oxidizing Substances and Organic Peroxides
|
| 2.2.5.1 |
Scope
|
| 2.2.5.1.1 |
Class 5 substances include oxidizing substances (Class 5.1) and organic peroxides (Class 5.2). Oxidizing substances are themselves not necessarily combustible. However, they may generally yield oxygen to support combustion of other materials. Organic peroxides are substances containing the bivalent –O–O– structure and may be considered derivatives of hydrogen peroxide, where one or both of the hydrogen atoms have been replaced by organic radicals. Organic peroxides are thermally unstable substances which may undergo exothermic self-accelerating decomposition.
|
| 2.2.5.2 |
Oxidizing substances under Class 5.1
|
| 2.2.5.2.1 |
Classification of Class 5.1 oxidizing substances
|
| 2.2.5.2.1.1 |
Class 5.1 substances with specific UN numbers shall be classified as stated in the DG List, including the packing groups, subsidiary hazards etc. However, for other substances which are not specified in the DG List but have properties possible to be classified as Class 5.1 DG, the corresponding test methods and criteria including advice on application of the tests are given in the United Nations Manual of Tests and Criteria for the classification. For solid substances, tests are performed to measure the potential for the substance to increase the burning rate or burning intensity of a combustible substance when the two are thoroughly mixed.16 Classification is based on the test results whether the mixture of substance and cellulose ignites and burns and the mean burning time (for the test O.1) or burning rate (for the test O.3) comparing with those of the reference mixtures.
16 The procedure is given in the United Nations Manual of Tests and Criteria, Part III, section 34.4.1 (test O.1) or alternatively in the section 34.4.3 (test O.3). Tests are conducted on the substance to be evaluated mixed with dry fibrous cellulose in mixing ratios of 1:1 and 4:1, by mass, of sample to cellulose, and compare with the burning characteristics of the mixtures.
|
| 2.2.5.2.1.2 |
A solid substance is classified in Class 5.1 if the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested ignites and burns, and exhibits a mean burning time equal to or less than the mean burning time of a 3:7 mixture (by mass) of potassium bromate and cellulose in the test O.1, or a mean burning rate equal to or greater than the mean burning rate of a 1:2 mixture (by mass) of calcium peroxide and cellulose in the test O.3.
|
| 2.2.5.2.1.3 |
For liquid substances, a test is performed to determine the potential for a liquid substance to increase the burning rate or burning intensity of a combustible substance or for spontaneous ignition to occur when the two are thoroughly mixed.17 Classification is based on test results whether the mixture of substance and cellulose spontaneously ignites, and the mean time taken for the pressure to rise from 690 kPa to 2070 kPa gauge (test O.2) comparing with those of the reference substances. 17 The procedure is given in the United Nations Manual of Tests and Criteria, Part III, 34.4.2 (test O.2). It measures the pressure rise time during combustion. Whether a liquid is an oxidizing substance of Class 5.1 and, if so, whether packing group I, II or III shall be assigned, is decided on the basis of the test result (see also Precedence of hazard characteristics in 2.1.2).
|
| 2.2.5.2.1.4 |
A liquid substance is classified in Class 5.1 if the 1:1 mixture, by mass, of substance and cellulose tested exhibits a mean pressure rise time less than or equal to the mean pressure rise time of a 1:1 mixture, by mass, of 65% aqueous nitric acid and cellulose.
|
| 2.2.5.2.2 |
Assignment of packing groups for Class 5.1 DG (oxidizing substances)
|
| 2.2.5.2.2.1 |
Solid oxidizing substances are assigned to a packing group according to the criteria based on the test results of the methods in the United Nations Manual of Tests and Criteria, Part III, Section 34 (test O.1) or alternatively (test O.3).
|
| 2.2.5.2.2.2 |
Liquid oxidizing substances are assigned to a packing group according to the criteria based on the test results of the method in the United Nations Manual of Tests and Criteria, Part III, Section 34 (test O.2). |
|
2.2.5.3 |
Organic peroxides under Class 5.2
|
|
2.2.5.3.1 |
Organic peroxides are liable to exothermic decomposition at normal or elevated temperatures. The decomposition can be initiated by heat, contact with impurities, friction or impact. The rate of decomposition increases with temperature and varies with the organic peroxide formulation. For certain organic peroxides the temperature shall be controlled. Some organic peroxides may decompose explosively, particularly if confined. This characteristic may be modified by the addition of diluents or by the use of appropriate packagings. Many organic peroxides burn vigorously.
|
|
2.2.5.3.2 |
Classification of organic peroxides
|
|
2.2.5.3.2.1 |
Any organic peroxide shall be considered for classification in Class 5.2, unless the organic peroxide formulation contains:
(a) not more than 1.0% available oxygen from the organic peroxides when containing not more than 1.0% hydrogen peroxide; or (b) not more than 0.5% available oxygen from the organic peroxides when containing more than 1.0% but not more than 7.0% hydrogen peroxide.
The available oxygen content (%) of an organic peroxide formulation is given by the formula: where:
ni = number of peroxygen groups per molecule of organic peroxide i ci = concentration (mass %) of organic peroxide i mi = molecular mass of organic peroxide i
|
|
2.2.5.3.2.2 |
Organic peroxides are classified into seven types according to the degree of danger they present. The types of organic peroxide range from type A, which is prohibited goods, to type G, which is not subject to the provisions for organic peroxides of Class 5.2. The classification of types B to F is directly related to the maximum quantity allowed in one packaging.
|
|
2.2.5.3.2.3 |
Organic peroxides permitted in packagings are listed in 2.2.5.4 of the Code. For each permitted substance listed, the generic entry of the DG List (UN 3101 to UN 3120) is assigned, appropriate subsidiary hazards and remarks providing relevant information are given.18
|
|
2.2.5.3.2.4 |
Mixtures of the listed formulations may be classified as the same type of organic peroxide as that of the most dangerous component under the conditions given for this type. However, as two stable components can form a thermally less stable mixture, the SADT of the mixture shall be determined19 and, if necessary, temperature control applied as required. For currently assigned organic peroxides, control and emergency temperatures are shown in the list in 2.2.5.4. Peroxide substances requiring temperature control shall refer to 7.3.7.2 in IMDG Code or 7.1.5.3 in Recommendations on the Transport of Dangerous Goods Model Regulations.
19 Test methods for determining the SADT are given in the United Nations Manual of Tests and Criteria, Part II, chapter 28. The test selected shall be conducted in a manner which is representative, both in size and material, of the package to be transported.
|
|
2.2.5.3.2.4.1 |
The following organic peroxides shall be subject to temperature control: (a) organic peroxides types B and C with an SADT ≤ 50°C; (b) organic peroxides type D showing a medium effect when heated under confinement20 with an SADT ≤ 50°C or showing a low or no effect when heated under confinement with an SADT ≤ 45°C; and (c) organic peroxides types E and F with an SADT ≤ 45°C. 20 Provisions for the determination of the effects of heating under confinement are given in the Manual of Tests and Criteria, Part II, Section 20 and Sub-section 28.4.
|
|
2.2.5.3.2.4.2 |
The actual temperature during handling may be lower than the control temperature but shall be selected so as to avoid dangerous separation of phases.
|
|
2.2.5.3.2.5 |
Samples of new organic peroxides or new formulations of currently assigned organic peroxides for which complete test data are not available and which are needed for further testing or evaluation may be assigned to one of the appropriate entries for ORGANIC PEROXIDE TYPE C provided the following conditions are met:
(b) the sample is appropriately packaged; and (c) the available data indicate that the control temperature, if any, is sufficiently low to prevent any dangerous decomposition and sufficiently high to prevent any dangerous phase separation.
|
|
2.2.5.3.2.6 |
Principles for classification of organic peroxides
|
|
2.2.5.3.2.6.1 |
Any organic peroxide formulation shall be regarded as possessing explosive properties when, in laboratory testing, the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement. These decisive properties shall be determined experimentally21 by the competent authority of the country of origin on the basis of a test report. The applicable classification procedures, test methods and criteria, and an example of a suitable test report, are given in the United Nations Manual of Tests and Criteria, Part II. The statement of approval shall contain the classification and other relevant conditions. Such statement of approval and the corresponding test report shall be provided as proof upon request.
21 Suitable test methods with pertinent evaluation criteria are given in the United Nations Manual of Tests and Criteria, Part II.
|
|
2.2.5.3.2.7 |
Desensitization of organic peroxides |
|
|
|
|
2.2.5.3.2.7.1 |
For safety reason, organic peroxides are in many cases desensitized by organic liquids or solids, inorganic solids or water. Where a percentage of a substance is stipulated, this refers to the percentage by mass, rounded to the nearest whole number. In general, desensitization shall be such that, in case of spillage, the organic peroxide will not concentrate to a dangerous extent.
|
|
2.2.5.3.2.7.2 |
Unless otherwise stated for the individual organic peroxide formulation, the following definition(s) shall apply to diluents used for desensitization:
(a) diluents type A are organic liquids which are compatible with the organic peroxide and which have a boiling point of not less than 150°C. Type A diluents may be used for desensitizing all organic peroxides. (b) diluents type B are organic liquids which are compatible with the organic peroxide and which have a boiling point of less than 150°C but not less than 60°C and a flash-point of not less than 5°C. Type B diluents may be used for desensitization of all organic peroxides provided that the boiling point of the liquid is at least 60°C higher than the SADT in a 50 kg package.
|
|
2.2.5.3.2.7.3 |
Diluents, other than type A or type B, may be added to organic peroxide formulations as listed in 2.2.5.4 provided that they are compatible. However, replacement of all or part of a type A or type B diluent by another diluent with differing properties requires that the organic peroxide formulation be re-assessed in accordance with the normal acceptance procedure for Class 5.2.
|
|
2.2.5.3.2.7.4 |
Water may only be used for the desensitization of organic peroxides which are listed in 2.2.5.4 or in the competent authority decision according to 2.2.5.3.2.6.1 as being “with water” or “as a stable dispersion in water”.
|
|
2.2.5.3.2.7.5 |
Organic and inorganic solids may be used for desensitization of organic peroxides provided that they are compatible.
|
|
2.2.5.3.2.7.6 |
Compatible liquids and solids are those which have no detrimental influence on the thermal stability and hazard type of the organic peroxide formulation.
|
|
2.2.5.4 |
List of currently assigned organic peroxides
Note 1: In the column “Packing Method”, codes “OP1” to “OP8” refer to packing methods in basic packing instruction BP520. |
|
ORGANIC PEROXIDE |
Concentration (%) |
Diluent Type A (%) |
Diluent Type B (%) |
Inert Solid (%) |
Water (%) |
Packing Method (BP520) |
Control Temperature (°C) |
Emergency Temperature (°C) |
UN |
Subsidiary Hazards and Remarks |
|
ACETYL ACETONE PEROXIDE |
≤ 42 |
≥ 48 |
|
|
≥8 |
OP7 |
|
|
3105 |
(2) |
|
ACETYL ACETONE PEROXIDE |
≤ 32 as a paste |
|
|
|
|
OP7 |
|
|
3106 |
(20) |
|
ACETYL CYCLOHEXANESULPHONYL PEROXIDE |
≤ 82 |
|
|
|
≥ 12 |
OP4 |
-10 |
0 |
3112 |
|
|
ACETYL CYCLOHEXANESULPHONYL PEROXIDE |
≤ 32 |
|
≥ 68 |
|
|
OP7 |
-10 |
0 |
3115 |
|
|
tert-AMYL HYDROPEROXIDE |
≤ 88 |
≥ 6 |
|
|
≥ 6 |
OP8 |
|
|
3107 |
|
|
tert-AMYL PEROXYACETATE |
≤ 62 |
≥ 38 |
|
|
|
OP7 |
|
|
3105 |
|
|
tert-AMYL PEROXYBENZOATE |
≤ 100 |
|
|
|
|
OP5 |
|
|
3103 |
|
|
tert-AMYL PEROXY-2-ETHYLHEXANOATE |
≤ 100 |
|
|
|
|
OP7 |
+20 |
+25 |
3115 |
|
|
tert-AMYL PEROXY-2-ETHYLHEXYL CARBONATE |
≤ 100 |
|
|
|
|
OP7 |
|
|
3105 |
|
|
tert-AMYL PEROXY ISOPROPYL CARBONATE |
≤ 77 |
≥ 23 |
|
|
|
OP5 |
|
|
3103 |
|
|
tert-AMYL PEROXYNEODECANOATE |
≤ 77 |
|
≥ 23 |
|
|
OP7 |
0 |
+10 |
3115 |
|
|
tert-AMYL PEROXYNEODECANOATE |
≤ 47 |
≥ 53 |
|
|
|
OP8 |
0 |
+10 |
3119 |
|
|
tert-AMYL PEROXYPIVALATE |
≤ 77 |
|
≥ 23 |
|
|
OP5 |
+10 |
+5 |
3113 |
|
|
tert-AMYL PEROXY-3,5,5-TRIMETHYLHEXANOATE |
≤ 100 |
|
|
|
|
OP7 |
|
|
3105 |
|
|
tert-BUTYL CUMYL PEROXIDE |
> 42 – 100 |
|
|
|
|
OP8 |
|
|
3109 |
|
|
tert-BUTYL CUMYL PEROXIDE |
≤ 52 |
|
|
≥ 48 |
|
OP8 |
|
|
3108 |
|
|
n-BUTYL 4,4-DI-(tert-BUTYLPEROXY)VALERATE |
> 52 – 100 |
|
|
|
|
OP5 |
|
|
3103 |
|
|
n-BUTYL 4,4-DI-(tert-BUTYLPEROXY)VALERATE |
≤ 52 |
|
|
≥ 48 |
|
OP8 |
|
|
3108 |
|
|
tert-BUTYL HYDROPEROXIDE |
>79 – 90 |
|
|
|
≥ 10 |
OP5 |
|
|
3103 |
(13) |
|
tert-BUTYL HYDROPEROXIDE |
≤ 80 |
≥ 20 |
|
|
|
OP7 |
|
|
3105 |
(4) (13) |
|
tert-BUTYL HYDROPEROXIDE |
≤ 79 |
|
|
|
> 14 |
OP8 |
|
|
3107 |
(13) (23) |
|
tert-BUTYL HYDROPEROXIDE |
≤ 72 |
|
|
|
≥ 28 |
OP8 |
|
|
3109 |
(13) |
|
tert-BUTYL HYDROPEROXIDE + DI-tert-BUTYLPEROXIDE |
< 82 + > 9 |
|
|
|
≥ 7 |
OP5 |
|
|
3103 |
(13) |
|
tert-BUTYL MONOPEROXYMALEATE |
> 52 – 100 |
|
|
|
|
OP5 |
|
|
3102 |
|
|
tert-BUTYL MONOPEROXYMALEATE |
≤ 52 |
≥ 48 |
|
|
|
OP6 |
|
|
3103 |
|
|
tert-BUTYL MONOPEROXYMALEATE |
≤ 52 |
|
|
≥ 48 |
|
OP8 |
|
|
3108 |
|
|
tert-BUTYL MONOPEROXYMALEATE |
≤ 52 as a paste |
|
|
|
|
OP8 |
|
|
3108 |
|
|
tert-BUTYL PEROXYACETATE |
> 52 – 77 |
≥ 23 |
|
|
|
OP5 |
|
|
3101 |
|
|
tert-BUTYL PEROXYACETATE |
> 32 – 52 |
≥ 48 |
|
|
|
OP6 |
|
|
3103 |
|
|
tert-BUTYL PEROXYACETATE |
≤ 32 |
|
≥ 68 |
|
|
OP8 |
|
|
3109 |
|
|
tert-BUTYL PEROXYBENZOATE |
> 77 – 100 |
|
|
|
|
OP5 |
|
|
3103 |
|
|
tert-BUTYL PEROXYBENZOATE |
> 52 – 77 |
≥ 23 |
|
|
|
OP7 |
|
|
3105 |
|
|
tert-BUTYL PEROXYBENZOATE |
≤ 52 |
|
|
≥ 48 |
|
OP7 |
|
|
3106 |
|
|
tert-BUTYL PEROXYBUTYL FUMARATE |
≤ 52 |
≥ 48 |
|
|
|
OP7 |
|
|
3105 |
|
|
tert-BUTYL PEROXYCROTONATE |
≤ 77 |
≥ 23 |
|
|
|
OP7 |
|
|
3105 |
|
|
tert-BUTYL PEROXYDIETHYLACETATE |
≤ 100 |
|
|
|
|
OP5 |
+20 |
+25 |
3113 |
|
|
tert-BUTYL PEROXY-2-ETHYLHEXANOATE |
> 52 – 100 |
|
|
|
|
OP6 |
+20 |
+25 |
3113 |
|
|
tert-BUTYL PEROXY-2-ETHYLHEXANOATE |
> 32 – 52 |
|
≥ 48 |
|
|
OP8 |
+30 |
+35 |
3117 |
|
|
tert-BUTYL PEROXY-2-ETHYLHEXANOATE |
≤ 52 |
|
|
≥ 48 |
|
OP8 |
+20 |
+25 |
3118 |
|
|
tert-BUTYL PEROXY-2-ETHYLHEXANOATE |
≤ 32 |
|
≥ 68 |
|
|
OP8 |
+40 |
+45 |
3119 |
|
|
tert-BUTYL PEROXY-2-ETHYLHEXANOATE + 2,2-DI-(tert- BUTYLPEROXY)BUTANE |
≤ 12 + ≤ 14 |
≥ 14 |
|
≥ 60 |
|
OP7 |
|
|
3106 |
|
|
tert-BUTYL PEROXY-2-ETHYLHEXANOATE + 2,2-DI-(tert- BUTYLPEROXY)BUTANE |
≤ 31 + ≤ 36 |
|
≥ 33 |
|
|
OP7 |
+35 |
+40 |
3115 |
|
|
tert-BUTYL PEROXY-2-ETHYLHEXYLCARBONATE |
≤ 100 |
|
|
|
|
OP7 |
|
|
3105 |
|
|
tert-BUTYL PEROXYISOBUTYRATE |
> 52 – 77 |
|
≥ 23 |
|
|
OP5 |
+15 |
+20 |
3111 |
|
|
tert-BUTYL PEROXYISOBUTYRATE |
≤ 52 |
|
≥ 48 |
|
|
OP7 |
+15 |
+20 |
3115 |
|
|
tert-BUTYL PEROXY ISOPROPYLCARBONATE |
≤ 77 |
≥ 23 |
|
|
|
OP5 |
|
|
3103 |
|
|
1-(2-tert-BUTYLPEROXYISOPROPYL)-3-ISOPROPENYLBENZENE |
≤ 77 |
≥ 23 |
|
|
|
OP7 |
|
|
3105 |
|
|
1-(2-tert-BUTYLPEROXYISOPROPYL)-3-ISOPROPENYLBENZENE |
≤ 42 |
|
|
≥ 58 |
|
OP8 |
|
|
3108 |
|
|
tert-BUTYL PEROXY-2-METHYLBENZOATE |
≤ 100 |
|
|
|
|
OP5 |
|
|
3103 |
|
|
tert-BUTYL PEROXYNEODECANOATE |
> 77 – 100 |
|
|
|
|
OP7 |
–5 |
+5 |
3115 |
|
|
tert-BUTYL PEROXYNEODECANOATE |
≤ 77 |
|
≥ 23 |
|
|
OP7 |
0 |
+10 |
3115 |
|
|
tert-BUTYL PEROXYNEODECANOATE |
≤ 52 as a stable dispersion in water |
|
|
|
|
OP8 |
0 |
+10 |
3119 |
|
|
tert-BUTYL PEROXYNEODECANOATE |
≤ 42 as a stable dispersion in water (frozen) |
|
|
|
|
OP8 |
0 |
+10 |
3118 |
|
|
tert-BUTYL PEROXYNEODECANOATE |
≤ 32 |
≥ 68 |
|
|
|
OP8 |
0 |
+10 |
3119 |
|
|
tert-BUTYL PEROXYNEOHEPTANOATE |
≤ 77 |
≥ 23 |
|
|
|
OP7 |
0 |
+10 |
3115 |
|
|
tert-BUTYL PEROXYNEOHEPTANOATE |
≤ 42 as a stable dispersion in water |
|
|
|
|
OP8 |
0 |
+10 |
3117 |
|
|
tert-BUTYL PEROXYPIVALATE |
> 67 – 77 |
≥ 23 |
|
|
|
OP5 |
0 |
+10 |
3113 |
|
|
tert-BUTYL PEROXYPIVALATE |
> 27 – 67 |
|
≥ 33 |
|
|
OP7 |
0 |
+10 |
3115 |
|
|
tert-BUTYL PEROXYPIVALATE |
≤ 27 |
|
≥ 73 |
|
|
OP8 |
+30 |
+35 |
3119 |
|
|
tert-BUTYL PEROXY STEARYLCARBONATE |
≤ 100 |
|
|
|
|
OP7 |
|
|
3106 |
|
|
tert-BUTYL PEROXY-3,5,5-TRIMETHYLHEXANOATE |
> 37 – 100 |
|
|
|
|
OP7 |
|
|
3105 |
|
|
tert-BUTYL PEROXY-3,5,5-TRIMETHYLHEXANOATE |
≤ 42 |
|
|
≥ 58 |
|
OP7 |
|
|
3106 |
|
|
tert-BUTYL PEROXY-3,5,5-TRIMETHYLHEXANOATE |
≤ 37 |
|
≥ 63 |
|
|
OP8 |
|
|
3109 |
|
|
3-CHLOROPEROXYBENZOIC ACID |
> 57 – 86 |
|
|
≥ 14 |
|
OP1 |
|
|
3102 |
|
|
3-CHLOROPEROXYBENZOIC ACID |
≤ 57 |
|
|
≥ 3 |
≥ 40 |
OP7 |
|
|
3106 |
|
|
3-CHLOROPEROXYBENZOIC ACID |
≤ 77 |
|
|
≥ 6 |
≥ 17 |
OP7 |
|
|
3106 |
|
|
CUMYL HYDROPEROXIDE |
> 90 - 98 |
≤ 10 |
|
|
|
OP8 |
|
|
3107 |
(13) |
|
CUMYL HYDROPEROXIDE |
≤ 90 |
≥ 10 |
|
|
|
OP8 |
|
|
3109 |
(13) (18) |
|
CUMYL PEROXYNEODECANOATE |
≤ 87 |
≥ 13 |
|
|
|
OP7 |
– 10 |
0 |
3115 |
|
|
CUMYL PEROXYNEODECANOATE |
≤ 77 |
|
≥ 23 |
|
|
OP7 |
–10 |
0 |
3115 |
|
|
CUMYL PEROXYNEODECANOATE |
≤ 52 as a stable dispersion in water |
|
|
|
|
OP8 |
–10 |
0 |
3119 |
|
|
CUMYL PEROXYNEOHEPTANOATE |
≤ 77 |
≥ 23 |
|
|
|
OP7 |
–10 |
0 |
3115 |
|
|
CUMYL PEROXYPIVALATE |
≤ 77 |
|
≥ 23 |
|
|
OP7 |
–5 |
+5 |
3115 |
|
|
CYCLOHEXANONE PEROXIDE(S) |
≤ 91 |
|
|
|
≥ 9 |
OP6 |
|
|
3104 |
(13) |
|
CYCLOHEXANONE PEROXIDE(S) |
≤ 72 |
≥ 28 |
|
|
|
OP7 |
|
|
3105 |
(5) |
|
CYCLOHEXANONE PEROXIDE(S) |
≤ 72 as a paste |
|
|
|
|
OP7 |
|
|
3106 |
(5) (20) |
|
CYCLOHEXANONE PEROXIDE(S) |
≤ 32 |
|
|
≥ 68 |
|
|
|
|
Exempt |
(29) |
|
([3R-(3R,5aS,6S,8aS,9R,10R,12S,12aR**)]-DECAHYDRO-10-METHOXY-3,6,9-TRIMETHYL-3,12-EPOXY-12H-PYRANO[4,3-j]-1,2-BENZODIOXEPIN) |
≤ 100 |
|
|
|
|
OP7 |
|
|
3106 |
|
|
DIACETONE ALCOHOL PEROXIDES |
≤ 57 |
|
≥ 26 |
|
≥ 8 |
OP7 |
+40 |
+45 |
3115 |
(6) |
|
DIACETYL PEROXIDE |
≤ 27 |
|
≥ 73 |
|
|
OP7 |
+20 |
+25 |
3115 |
(7) (13) |
|
DI-tert-AMYL PEROXIDE |
≤ 100 |
|
|
|
|
OP8 |
|
|
3107 |
|
|
2,2-DI-(tert-AMYLPEROXY)BUTANE |
≤ 57 |
≥ 43 |
|
|
|
OP7 |
|
|
3105 |
|
|
1,1-DI-(tert-AMYLPEROXY)CYCLOHEXANE |
≤ 82 |
≥ 18 |
|
|
|
OP6 |
|
|
3103 |
|
|
DIBENZOYL PEROXIDE |
> 52 – 100 |
|
|
≤ 48 |
|
OP2 |
|
|
3102 |
|
|
DIBENZOYL PEROXIDE |
> 77 – 94 |
|
|
|
≥ 6 |
OP4 |
|
|
3102 |
|
|
DIBENZOYL PEROXIDE |
≤ 77 |
|
|
|
≥ 23 |
OP6 |
|
|
3104 |
|
|
DIBENZOYL PEROXIDE |
≤ 62 |
|
|
≥ 28 |
≥ 10 |
OP7 |
|
|
3106 |
|
|
DIBENZOYL PEROXIDE |
> 52 – 62 as a paste |
|
|
|
|
OP7 |
|
|
3106 |
(20) |
|
DIBENZOYL PEROXIDE |
> 35 – 52 |
|
|
≥ 48 |
|
OP7 |
|
|
3106 |
|
|
DIBENZOYL PEROXIDE |
> 36 – 42 |
≥ 18 |
|
|
≤ 40 |
OP8 |
|
|
3107 |
|
|
DIBENZOYL PEROXIDE |
≤ 56.5 as a paste |
|
|
|
≥ 15 |
OP8 |
|
|
3108 |
|
|
DIBENZOYL PEROXIDE |
≤ 52 as a paste |
|
|
|
|
OP8 |
|
|
3108 |
(20) |
|
DIBENZOYL PEROXIDE |
≤ 42 as a stable dispersion in water |
|
|
|
|
OP8 |
|
|
3109 |
|
|
DIBENZOYL PEROXIDE |
≤ 35 |
|
|
≥ 65 |
|
|
|
|
Exempt |
(29) |
|
DI-(4-tert-BUTYLCYCLOHEXYL) PEROXYDICARBONATE |
≤ 100 |
|
|
|
|
OP6 |
+30 |
+35 |
3114 |
|
|
DI-(4-tert-BUTYLCYCLOHEXYL) PEROXYDICARBONATE |
≤ 42 as a stable dispersion in water |
|
|
|
|
OP8 |
+30 |
+35 |
3119 |
|
|
DI-(4-tert-BUTYLCYCLOHEXYL) PEROXYDICARBONATE |
≤ 42 as a paste |
|
|
|
|
OP8 |
+35 |
+40 |
3118 |
|
|
DI-tert-BUTYL PEROXIDE |
> 52 – 100 |
|
|
|
|
OP8 |
|
|
3107 |
|
|
DI-tert-BUTYL PEROXIDE |
≤ 52 |
|
≥ 48 |
|
|
OP8 |
|
|
3109 |
(25) |
|
DI-tert-BUTYL PEROXYAZELATE |
≤ 52 |
≥ 48 |
|
|
|
OP7 |
|
|
3105 |
|
|
2,2-DI-(tert-BUTYLPEROXY)BUTANE |
≤ 52 |
≥ 48 |
|
|
|
OP6 |
|
|
3103 |
|
|
1,6-DI-(tert-BUTYLPEROXYCARBONYLOXY) HEXANE |
≤ 72 |
≥ 28 |
|
|
|
OP5 |
|
|
3103 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
> 80 – 100 |
|
|
|
|
OP5 |
|
|
3101 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
≤ 72 |
|
≥ 28 |
|
|
OP5 |
|
|
3103 |
(30) |
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
> 52 – 80 |
≥ 20 |
|
|
|
OP5 |
|
|
3103 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
> 42 – 52 |
≥ 48 |
|
|
|
OP7 |
|
|
3105 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
≤ 42 |
≥ 13 |
|
≥ 45 |
|
OP7 |
|
|
3106 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
≤ 42 |
≥ 58 |
|
|
|
OP8 |
|
|
3109 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
≤ 27 |
≥ 25 |
|
|
|
OP8 |
|
|
3107 |
(21) |
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
≤ 13 |
≥ 13 |
≥ 74 |
|
|
OP8 |
|
|
3109 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE + tert-BUTYL PEROXY-2-ETHYLHEXANOATE |
≤ 43 + ≤ 16 |
≥ 41 |
|
|
|
OP7 |
|
|
3105 |
|
|
DI-n-BUTYL PEROXYDICARBONATE |
> 27 – 52 |
|
≥ 48 |
|
|
OP7 |
–15 |
–5 |
3115 |
|
|
DI-n-BUTYL PEROXYDICARBONATE |
≤ 42 as a stable dispersion in water (frozen) |
|
|
|
|
OP8 |
–15 |
–5 |
3118 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
≤ 42 as a stable dispersion in water (frozen) |
|
|
|
|
OP8 |
–15 |
–5 |
3118 |
|
|
1,1-DI-(tert-BUTYLPEROXY)CYCLOHEXANE |
≤ 27 |
|
≥ 73 |
|
|
OP8 |
–10 |
0 |
3117 |
|
|
DI-sec-BUTYL PEROXYDICARBONATE |
> 52 – 100 |
|
|
|
|
OP4 |
–20 |
–10 |
3113 |
|
|
DI-sec-BUTYL PEROXYDICARBONATE |
≤ 52 |
|
≥ 48 |
|
|
OP7 |
–15 |
–5 |
3115 |
|
|
DI-(tert-BUTYLPEROXYISOPROPYL)BENZENE(S) |
> 42 – 100 |
|
|
≤ 57 |
|
OP7 |
|
|
3106 |
|
|
DI-(tert-BUTYLPEROXYISOPROPYL)BENZENE(S) |
≤ 42 |
|
|
≥ 58 |
|
|
|
|
Exempt |
(29) |
|
DI-(tert-BUTYLPEROXY)PHTHALATE |
> 42 – 52 |
≥ 48 |
|
|
|
OP7 |
|
|
3105 |
|
|
DI-(tert-BUTYLPEROXY)PHTHALATE |
≤ 52 as a paste |
|
|
|
|
OP7 |
|
|
3106 |
(20) |
|
DI-(tert-BUTYLPEROXY)PHTHALATE |
≤ 42 |
≥ 58 |
|
|
|
OP8 |
|
|
3107 |
|
|
2,2-DI-(tert-BUTYLPEROXY)PROPANE |
≤ 52 |
≥ 48 |
|
|
|
OP7 |
|
|
3105 |
|
|
2,2-DI-(tert-BUTYLPEROXY)PROPANE |
≤ 42 |
≥ 13 |
|
≥ 45 |
|
OP7 |
|
|
3106 |
|
|
1,1-DI-(tert-BUTYLPEROXY)-3,3,5-TRIMETHYLCYCLOHEXANE |
> 90 – 100 |
|
|
|
|
OP5 |
|
|
3101 |
|
|
1,1-DI-(tert-BUTYLPEROXY)-3,3,5-TRIMETHYLCYCLOHEXANE |
≤ 90 |
|
≥ 10 |
|
|
OP5 |
|
|
3103 |
(30) |
|
1,1-DI-(tert-BUTYLPEROXY)-3,3,5-TRIMETHYLCYCLOHEXANE |
> 57 – 90 |
≥ 10 |
|
|
|
OP5 |
|
|
3103 |
|
|
1,1-DI-(tert-BUTYLPEROXY)-3,3,5-TRIMETHYLCYCLOHEXANE |
≤ 77 |
|
≥ 23 |
|
|
OP5 |
|
|
3103 |
|
|
1,1-DI-(tert-BUTYLPEROXY)-3,3,5-TRIMETHYLCYCLOHEXANE |
≤ 57 |
|
|
≥ 43 |
|
OP8 |
|
|
3110 |
|
|
1,1-DI-(tert-BUTYLPEROXY)-3,3,5-TRIMETHYLCYCLOHEXANE |
≤ 57 |
≥ 43 |
|
|
|
OP8 |
|
|
3107 |
|
|
1,1-DI-(tert-BUTYLPEROXY)-3,3,5-TRIMETHYLCYCLOHEXANE |
≤ 32 |
≥ 26 |
≥ 42 |
|
|
OP8 |
|
|
3107 |
|
|
DICETYL PEROXYDICARBONATE |
≤ 100 |
|
|
|
|
OP8 |
+30 |
+35 |
3120 |
|
|
DICETYL PEROXYDICARBONATE |
≤ 42 as a stable dispersion in water |
|
|
|
|
OP8 |
+30 |
+35 |
3119 |
|
|
DI-4-CHLOROBENZOYL PEROXIDE |
≤ 77 |
|
|
|
≥ 23 |
OP5 |
|
|
3102 |
|
|
DI-4-CHLOROBENZOYL PEROXIDE |
≤ 52 as a paste |
|
|
|
|
OP7 |
|
|
3106 |
(20) |
|
DI-4-CHLOROBENZOYL PEROXIDE |
≤ 32 |
|
|
≥ 68 |
|
|
|
|
Exempt |
(29) |
|
DICUMYL PEROXIDE |
> 52 - 100 |
|
|
|
|
OP8 |
|
|
3110 |
(12) |
|
DICUMYL PEROXIDE |
≤ 52 |
|
|
≥ 48 |
|
|
|
|
Exempt |
(29) |
|
DICYCLOHEXYL PEROXYDICARBONATE |
> 91 - 100 |
|
|
|
|
OP3 |
+10 |
+15 |
3112 |
|
|
DICYCLOHEXYL PEROXYDICARBONATE |
≤ 91 |
|
|
|
≥ 9 |
OP5 |
+10 |
+15 |
3114 |
|
|
DICYCLOHEXYL PEROXYDICARBONATE |
≤ 42 as a stable dispersion in water |
|
|
|
|
OP8 |
+15 |
+20 |
3119 |
|
|
DIDECANOYL PEROXIDE |
≤ 100 |
|
|
|
|
OP6 |
+30 |
+35 |
3114 |
|
|
2,2-DI-(4,4-DI-(tert-BUTYLPEROXY)CYCLOHEXYL)-PROPANE |
≤ 42 |
|
|
≥ 58 |
|
OP7 |
|
|
3106 |
|
|
2,2-DI-(4,4-DI-(tert-BUTYLPEROXY)CYCLOHEXYL)-PROPANE |
≤ 22 |
|
≥ 78 |
|
|
OP8 |
|
|
3107 |
|
|
DI-2,4-DICHLOROBENZOYL PEROXIDE |
≤ 77 |
|
|
|
≥ 23 |
OP5 |
|
|
3102 |
|
|
DI-2,4-DICHLOROBENZOYL PEROXIDE |
≤ 52 as a paste |
|
|
|
|
OP8 |
+ 20 |
+ 25 |
3118 |
|
|
DI-2,4-DICHLOROBENZOYL PEROXIDE |
≤ 52 as a paste with silicon oil |
|
|
|
|
OP7 |
|
|
3106 |
|
|
DI-(2-ETHOXYETHYL) PEROXYDICARBONATE |
≤ 52 |
|
≥ 48 |
|
|
OP7 |
-10 |
0 |
3115 |
|
|
DI-(2-ETHYLHEXYL) PEROXYDICARBONATE |
> 77 - 100 |
|
|
|
|
OP5 |
-20 |
-10 |
3113 |
|
|
DI-(2-ETHYLHEXYL) PEROXYDICARBONATE |
≤ 77 |
|
≥ 23 |
|
|
OP7 |
-15 |
-5 |
3115 |
|
|
DI-(2-ETHYLHEXYL) PEROXYDICARBONATE |
≤ 62 as a stable dispersion in water |
|
|
|
|
OP8 |
-15 |
-5 |
3119 |
|
|
DI-(2-ETHYLHEXYL) PEROXYDICARBONATE |
≤ 52 as a stable dispersion in water (frozen) |
|
|
|
|
OP8 |
-15 |
-5 |
3120 |
|
|
2,2-DIHYDROPEROXYPROPANE |
≤ 27 |
|
|
≥ 73 |
|
OP5 |
|
|
3102 |
|
|
DI-(1-HYDROXYCYCLOHEXYL) PEROXIDE |
≤ 100 |
|
|
|
|
OP7 |
|
|
3106 |
|
|
DIISOBUTYRYL PEROXIDE |
> 32 - 52 |
|
≥ 48 |
|
|
OP5 |
-20 |
-10 |
3111 |
|
|
DIISOBUTYRYL PEROXIDE |
≤ 42 (as a stable dispersion in water) |
|
|
|
|
OP8 |
-20 |
-10 |
3119 |
|
|
DIISOBUTYRYL PEROXIDE |
≤ 32 |
|
≥ 68 |
|
|
OP7 |
-20 |
-10 |
3115 |
|
|
DIISOPROPYLBENZENE DIHYDROPEROXIDE |
≤ 82 |
≥ 5 |
|
|
≥ 5 |
OP7 |
|
|
3106 |
(24) |
|
DIISOPROPYL PEROXYDICARBONATE |
> 52 - 100 |
|
|
|
|
OP2 |
-15 |
-5 |
3112 |
|
|
DIISOPROPYL PEROXYDICARBONATE |
≤ 52 |
|
≥ 48 |
|
|
OP7 |
-20 |
-10 |
3115 |
|
|
DIISOPROPYL PEROXYDICARBONATE |
≤ 32 |
≥ 68 |
|
|
|
OP7 |
-15 |
-5 |
3115 |
|
|
DILAUROYL PEROXIDE |
≤ 100 |
|
|
|
|
OP7 |
|
|
3106 |
|
|
DILAUROYL PEROXIDE |
≤ 42 as a stable dispersion in water |
|
|
|
|
OP8 |
|
|
3109 |
|
|
DI-(3-METHOXYBUTYL) PEROXYDICARBONATE |
≤ 52 |
|
≥ 48 |
|
|
OP7 |
-5 |
+5 |
3115 |
|
|
DI-(2-METHYLBENZOYL) PEROXIDE |
≤ 87 |
|
|
|
≥ 13 |
OP5 |
+30 |
+35 |
3112 |
|
|
DI-(3-METHYLBENZOYL) PEROXIDE + BENZOYL (3- METHYLBENZOYL) PEROXIDE + DIBENZOYL PEROXIDE |
≤ 20 + ≤ 18 + ≤ 4 |
|
≥ 58 |
|
|
OP7 |
+35 |
+40 |
3115 |
|
|
DI-(4-METHYLBENZOYL) PEROXIDE |
≤ 52 as a paste with silicon oil |
|
|
|
|
OP7 |
|
|
3106 |
|
|
2,5-DIMETHYL-2,5-DI-(BENZOYLPEROXY)HEXANE |
> 82-100 |
|
|
|
|
OP5 |
|
|
3102 |
|
|
2,5-DIMETHYL-2,5-DI-(BENZOYLPEROXY)HEXANE |
≤ 82 |
|
|
≥ 18 |
|
OP7 |
|
|
3106 |
|
|
2,5-DIMETHYL-2,5-DI-(BENZOYLPEROXY)HEXANE |
≤ 82 |
|
|
|
≥ 18 |
OP5 |
|
|
3104 |
|
|
2,5-DIMETHYL-2,5-DI-(tert-BUTYLPEROXY)HEXANE |
> 90 - 100 |
|
|
|
|
OP5 |
|
|
3103 |
|
|
2,5-DIMETHYL-2,5-DI-(tert-BUTYLPEROXY)HEXANE |
> 52 - 90 |
≥ 10 |
|
|
|
OP7 |
|
|
3105 |
|
|
2,5-DIMETHYL-2,5-DI-(tert-BUTYLPEROXY)HEXANE |
≤ 77 |
|
|
≥ 23 |
|
OP8 |
|
|
3108 |
|
|
2,5-DIMETHYL-2,5-DI-(tert-BUTYLPEROXY)HEXANE |
≤ 52 |
≥ 48 |
|
|
|
OP8 |
|
|
3109 |
|
|
2,5-DIMETHYL-2,5-DI-(tert-BUTYLPEROXY)HEXANE |
≤ 47 as a paste |
|
|
|
|
OP8 |
|
|
3108 |
|
|
2,5-DIMETHYL-2,5-DI-(tert-BUTYLPEROXY)HEXYNE-3 |
> 86 - 100 |
|
|
|
|
OP5 |
|
|
3101 |
|
|
2,5-DIMETHYL-2,5-DI-(tert-BUTYLPEROXY)HEXYNE-3 |
>52 - 86 |
≥ 14 |
|
|
|
OP5 |
|
|
3103 |
(26) |
|
2,5-DIMETHYL-2,5-DI-(tert-BUTYLPEROXY)HEXYNE-3 |
≤ 52 |
|
|
≥ 48 |
|
OP7 |
|
|
3106 |
|
|
2,5-DIMETHYL-2,5-DI-(2-ETHYLHEXANOYLPEROXY)HEXANE |
≤ 100 |
|
|
|
|
OP5 |
+20 |
+25 |
3113 |
|
|
2,5-DIMETHYL-2,5-DIHYDROPEROXYHEXANE |
≤ 82 |
|
|
|
≥ 18 |
OP6 |
|
|
3104 |
|
|
2,5-DIMETHYL-2,5-DI-(3,5,5-TRIMETHYLHEXANOYLPEROXY) HEXANE |
≤ 77 |
≥ 23 |
|
|
|
OP7 |
|
|
3105 |
|
|
1,1-DIMETHYL-3-HYDROXYBUTYL PEROXYNEOHEPTANOATE |
≤ 52 |
≥ 48 |
|
|
|
OP8 |
0 |
+10 |
3117 |
|
|
DIMYRISTYL PEROXYDICARBONATE |
≤ 100 |
|
|
|
|
OP7 |
+20 |
+25 |
3116 |
|
|
DIMYRISTYL PEROXYDICARBONATE |
≤ 42 as a stable dispersion in water |
|
|
|
|
OP8 |
+20 |
+25 |
3119 |
|
|
DI-(2-NEODECANOYLPEROXYISOPROPYL)BENZENE |
≤ 52 |
≥ 48 |
|
|
|
OP7 |
-10 |
0 |
3115 |
|
|
DI-n-NONANOYL PEROXIDE |
≤ 100 |
|
|
|
|
OP7 |
0 |
+10 |
3116 |
|
|
DI-n-OCTANOYL PEROXIDE |
≤ 100 |
|
|
|
|
OP5 |
+10 |
+15 |
3114 |
|
|
DI-(2-PHENOXYETHYL) PEROXYDICARBONATE |
> 85 - 100 |
|
|
|
|
OP5 |
|
|
3102 |
|
|
DI-(2-PHENOXYETHYL) PEROXYDICARBONATE |
≤ 85 |
|
|
|
≥ 15 |
OP7 |
|
|
3106 |
|
|
DIPROPIONYL PEROXIDE |
≤ 27 |
|
≥ 73 |
|
|
OP8 |
+15 |
+20 |
3117 |
|
|
DI-n-PROPYL PEROXYDICARBONATE |
≤ 100 |
|
|
|
|
OP3 |
-25 |
-15 |
3113 |
|
|
DI-n-PROPYL PEROXYDICARBONATE |
≤ 77 |
|
≥ 23 |
|
|
OP5 |
-20 |
-10 |
3113 |
|
|
DISUCCINIC ACID PEROXIDE |
> 72 - 100 |
|
|
|
|
OP4 |
|
|
3102 |
(17) |
|
DISUCCINIC ACID PEROXIDE |
≤ 72 |
|
|
|
≥ 28 |
OP7 |
+10 |
+15 |
3116 |
|
|
DI-(3,5,5-TRIMETHYLHEXANOYL) PEROXIDE |
> 52 - 82 |
≥ 18 |
|
|
|
OP7 |
0 |
+10 |
3115 |
|
|
DI-(3,5,5-TRIMETHYLHEXANOYL) PEROXIDE |
≤ 52 as a stable dispersion in water |
|
|
|
|
OP8 |
+10 |
+15 |
3119 |
|
|
DI-(3,5,5-TRIMETHYLHEXANOYL) PEROXIDE |
> 38 - 52 |
≥ 48 |
|
|
|
OP8 |
+10 |
+15 |
3119 |
|
|
DI-(3,5,5-TRIMETHYLHEXANOYL) PEROXIDE |
≤ 38 |
≥ 62 |
|
|
|
OP8 |
+20 |
+25 |
3119 |
|
|
ETHYL 3,3-DI-(tert-AMYLPEROXY)BUTYRATE |
≤ 67 |
≥ 33 |
|
|
|
OP7 |
|
|
3105 |
|
|
ETHYL 3,3-DI-(tert-BUTYLPEROXY)BUTYRATE |
> 77 - 100 |
|
|
|
|
OP5 |
|
|
3103 |
|
|
ETHYL 3,3-DI-(tert-BUTYLPEROXY)BUTYRATE |
≤ 77 |
≥ 23 |
|
|
|
OP7 |
|
|
3105 |
|
|
ETHYL 3,3-DI-(tert-BUTYLPEROXY)BUTYRATE |
≤ 52 |
|
|
≥ 48 |
|
OP7 |
|
|
3106 |
|
|
1-(2-ETHYLHEXANOYLPEROXY)-1,3-DIMETHYLBUTYL PEROXYPIVALATE |
≤ 52 |
≥ 45 |
≥ 10 |
|
|
OP7 |
–20 |
–10 |
3115 |
|
|
tert-HEXYL PEROXYNEODECANOATE |
≤ 71 |
≥ 29 |
|
|
|
OP7 |
0 |
+10 |
3115 |
|
|
tert-HEXYL PEROXYPIVALATE |
≤ 72 |
|
≥ 28 |
|
|
OP7 |
+10 |
+15 |
3115 |
|
|
3-HYDROXY-1,1-DIMETHYLBUTYL PEROXYNEODECANOATE |
≤ 77 |
≥ 23 |
|
|
|
OP7 |
–5 |
+ 5 |
3115 |
|
|
3-HYDROXY-1,1-DIMETHYLBUTYL PEROXYNEODECANOATE |
≤ 52 |
≥ 48 |
|
|
|
OP8 |
–5 |
+ 5 |
3117 |
|
|
3-HYDROXY-1,1-DIMETHYLBUTYL PEROXYNEODECANOATE |
≤ 52 as a stable dispersion in water |
|
|
|
|
OP8 |
–5 |
+ 5 |
3119 |
|
|
ISOPROPYL sec-BUTYL PEROXYDICARBONATE |
≤ 32 |
≥ 38 |
|
|
|
OP7 |
–20 |
–10 |
3115 |
|
|
ISOPROPYL sec-BUTYL PEROXYDICARBONATE |
≤ 52 |
|
|
|
|
OP5 |
–20 |
–10 |
3111 |
|
|
ISOPROPYLCUMYL HYDROPEROXIDE |
≤ 72 |
≥ 28 |
|
|
|
OP8 |
|
|
3109 |
(13) |
|
p-MENTHYL HYDROPEROXIDE |
> 72 – 100 |
|
|
|
|
OP7 |
|
|
3105 |
(13) |
|
p-MENTHYL HYDROPEROXIDE |
≤ 72 |
≥ 28 |
|
|
|
OP8 |
|
|
3109 |
(27) |
|
METHYLCYCLOHEXANONE PEROXIDE(S) |
≤ 67 |
|
≥ 33 |
|
|
OP7 |
+35 |
+40 |
3115 |
|
|
METHYL ETHYL KETONE PEROXIDE(S) |
See remark (8) |
≥ 48 |
|
|
|
OP5 |
|
|
3101 |
(8) (13) |
|
METHYL ETHYL KETONE PEROXIDE(S) |
See remark (9) |
≥ 55 |
|
|
|
OP7 |
|
|
3105 |
(9) |
|
METHYL ETHYL KETONE PEROXIDE(S) |
See remark (10) |
≥ 60 |
|
|
|
OP8 |
|
|
3107 |
(10) |
|
METHYL ISOBUTYL KETONE PEROXIDE(S) |
≤ 62 |
≥ 19 |
|
|
|
OP7 |
|
|
3105 |
(22) |
|
METHYL ISOPROPYL KETONE PEROXIDE(S) |
See remark (31) |
≥ 70 |
|
|
|
OP8 |
|
|
3109 |
(31) |
|
ORGANIC PEROXIDE, LIQUID, SAMPLE |
|
|
|
|
|
OP2 |
|
|
3103 |
(11) |
|
ORGANIC PEROXIDE, LIQUID, SAMPLE, TEMPERATURE CONTROLLED |
|
|
|
|
|
OP2 |
|
|
3113 |
(11) |
|
ORGANIC PEROXIDE, SOLID, SAMPLE |
|
|
|
|
|
OP2 |
|
|
3104 |
(11) |
|
ORGANIC PEROXIDE, SOLID, SAMPLE, TEMPERATURE CONTROLLED |
|
|
|
|
|
OP2 |
|
|
3114 |
(11) |
|
3,3,5,7,7-PENTAMETHYL-1,2,4-TRIOXEPANE |
≤ 100 |
|
|
|
|
OP8 |
|
|
3107 |
|
|
PEROXYACETIC ACID, TYPE D, stabilized |
≤ 43 |
|
|
|
|
OP7 |
|
|
3105 |
(13) (14) (19) |
|
PEROXYACETIC ACID, TYPE E, stabilized |
≤ 43 |
|
|
|
|
OP8 |
|
|
3107 |
(13) (15) (19) |
|
PEROXYACETIC ACID, TYPE F, stabilized |
≤ 43 |
|
|
|
|
OP8 |
|
|
3109 |
(13) (16) (19) |
|
PEROXYLAURIC ACID |
≤ 100 |
|
|
|
|
OP8 |
+35 |
+40 |
3118 |
|
|
1-PHENYLETHYL HYDROPEROXIDE |
≤ 38 |
|
≥62 |
|
|
OP8 |
|
|
3109 |
|
|
PINANYL HYDROPEROXIDE |
> 56 – 100 |
|
|
|
|
OP7 |
|
|
3105 |
(13) |
|
PINANYL HYDROPEROXIDE |
≤ 56 |
≥ 44 |
|
|
|
OP8 |
|
|
3109 |
|
|
POLYETHER POLY-tert-BUTYLPEROXYCARBONATE |
≤ 52 |
|
≥ 48 |
|
|
OP8 |
|
|
3107 |
|
|
1,1,3,3-TETRAMETHYLBUTYL HYDROPEROXIDE |
≤ 100 |
|
|
|
|
OP7 |
|
|
3105 |
|
|
1,1,3,3-TETRAMETHYLBUTYL PEROXY-2 ETHYLHEXANOATE |
≤ 100 |
|
|
|
|
OP7 |
+15 |
+20 |
3115 |
|
|
1,1,3,3- TETRAMETHYLBUTYL PEROXYNEODECANOATE |
≤ 72 |
|
≥ 28 |
|
|
OP7 |
–5 |
+5 |
3115 |
|
|
1,1,3,3- TETRAMETHYLBUTYL PEROXYNEODECANOATE |
≤ 52 as a stable dispersion in water |
|
|
|
|
OP8 |
–5 |
+5 |
3119 |
|
|
1,1,3,3-TETRAMETHYLBUTYL PEROXYPIVALATE |
≤ 77 |
≥ 23 |
|
|
|
OP7 |
0 |
+10 |
3115 |
|
|
3,6,9-TRIETHYL-3,6,9-TRIMETHYL-1,4,7 TRIPEROXONANE |
≤ 42 |
≥ 58 |
|
|
|
OP7 |
|
|
3105 |
(28) |
|
3,6,9-TRIETHYL-3,6,9-TRIMETHYL-1,4,7 TRIPEROXONANE |
≤ 17 |
≥ 18 |
|
≥ 65 |
|
OP8 |
|
|
3110 |
|
Remarks:
|
(1) Diluent type B may always be replaced by diluent type A. The boiling point of diluent type B shall be at least 60°C higher than the SADT of the organic peroxide. (2) Available oxygen ≤ 4.7% |
| 2.2.6 |
Class 6.1 – Toxic Substances
|
||||||||||||||||||||
| 2.2.6.1 |
Scope
|
||||||||||||||||||||
| 2.2.6.1.1 |
Class 6.1 are toxic substances liable either to cause death or serious injury or to harm human health if swallowed or inhaled, or by skin contact.
|
||||||||||||||||||||
| 2.2.6.1.2 |
In general, toxic substances having specific UN numbers shall be classified as stated in the DG List, including the packing groups, subsidiary hazards etc. For the substances which are not specified in the DG List but possible to be classified as Class 6.1 DG, the three possible exposure routes of toxic substances i.e. are oral ingestion, dermal contact and inhalation of dusts, mists or vapours; the LD50 (median lethal dose) for acute oral toxicity, the LD50 for acute dermal toxicity, or the LC50 for acute toxicity on inhalation of the substances are examined for the classification of the substances under Class 6.1.
|
||||||||||||||||||||
| 2.2.6.1.3 |
When a substance exhibits a different order of toxicity by two or more routes of administration, the highest degree of danger indicated by the tests shall be assigned.
|
||||||||||||||||||||
| 2.2.6.1.4 |
The LD50 for the oral and dermal routes as well as LC50 for inhalation of dusts and mists are considered as the criteria for the three packing groups assignment as shown in the following table: |
||||||||||||||||||||
|
22 The criteria for inhalation toxicity of dusts and mists are based on LC50 data relating to one-hour exposures, and where such information is available it shall be used. However, where only LC50 data relating to four-hour exposures to dusts and mists are available, such figures can be multiplied by four and the product substituted in the above criteria, i.e. LC50 (four-hours) × 4 is considered the equivalent of LC50 (one-hour).
|
|||||||||||||||||||||
| 2.2.6.1.5 |
For liquids having toxic vapours of saturated vapour concentration “V” in ml/m3 air at 20°C and standard atmospheric pressure23, the assignment of their packing groups shall refer to 2.6.2.2.4.3 to 2.6.2.2.4.5 in IMDG Code or 2.6.2.2.4.3 to 2.6.2.2.4.5 in Recommendations on the Transport of Dangerous Goods Model Regulations.
23 The criteria for inhalation toxicity of vapours are based on LC50 data relating to one-hour exposures, and where such information is available, it shall be used. However, where only LC50 data relating to four-hour exposures to the vapours are available, such figures can be multiplied by two and the product substituted in the above criteria, i.e. LC50 (four-hours) × 2 is considered the equivalent of LC50 (one-hour).
|
||||||||||||||||||||
| 2.2.6.1.6 |
For mixtures of liquids that are toxic by inhalation, the following two cases can be considered in the packing group determination.
|
||||||||||||||||||||
| 2.2.6.1.6.1 |
If the LC50 data are available for each of the toxic substances comprising the mixture, 2.6.2.2.4.6 to 2.6.2.2.4.7 in IMDG Code or 2.6.2.2.4.6 to 2.6.2.2.4.7 in Recommendations on the Transport of Dangerous Goods Model Regulations may be applied for the calculation of the packing group.
|
||||||||||||||||||||
| 2.2.6.1.6.2 |
If the LC50 data on the toxic constituent substances of the mixtures of liquids that are toxic by inhalation are absence, the mixture may be assigned a packing group based on the simplified threshold toxicity tests in 2.6.2.2.4.8 in IMDG Code or 2.6.2.2.4.8 in Recommendations on the Transport of Dangerous Goods Model Regulations. It is noted that when these threshold tests are used, the most restrictive packing group shall be determined and used.
|
||||||||||||||||||||
| 2.2.6.2 |
Methods for determining oral and dermal toxicity of mixtures
|
||||||||||||||||||||
| 2.2.6.2.1 |
When classifying and assigning the appropriate packing group to mixtures in Class 6.1, it is necessary to determine the LD50 for acute oral and dermal toxicities of the mixture following 2.6.2.3.1 to 2.6.2.3.3 in IMDG Code or 2.6.2.3.1 to 2.6.2.3.3 in Recommendations on the Transport of Dangerous Goods Model Regulations.
|
||||||||||||||||||||
| 2.2.6.2.1.1 |
LD50 (median lethal dose) for acute oral toxicity is the statistically derived single dose of a substance that can be expected to cause death within 14 days in 50% of both male and female young adult albino rats when administered by the oral route. The LD50 value is expressed in terms of mass of test substance per mass of test animal (milligrams per kilogram).
|
||||||||||||||||||||
| 2.2.6.2.1.2 |
LD50 for acute dermal toxicity is that dose of the substance which, administered by continuous contact for 24 hours with the bare skin of the albino rabbit, is most likely to cause death within 14 days in one half of the animals tested. The number of animals tested shall be sufficient to give a statistically significant result and be in conformity with good pharmacological practices. The result is expressed in milligrams per kilogram body mass.
|
||||||||||||||||||||
| 2.2.6.2.1.3 |
LC50 for acute toxicity on inhalation is that concentration of vapour, mist or dust which, administered by continuous inhalation to both male and female young adult albino rats for one hour, is most likely to cause death within 14 days in one half of the animals tested. A solid substance shall be tested if at least 10% (by mass) of its total mass is likely to be dust in the respirable range, such as the aerodynamic diameter of that particle fraction is 10 microns or less. A liquid substance shall be tested if a mist is likely to be generated in a leakage of the containment. For both solid and liquid substances, more than 90% (by mass) of a specimen prepared for inhalation toxicity testing shall be in the respirable range as defined above. The result is expressed in milligrams per litre of air for dusts and mists or in millilitres per cubic metre of air (parts per million) for vapours. |
||||||||||||||||||||
| 2.2.7 |
(Reserved) |
| 2.2.8 |
Class 8 – Corrosive Substances
|
||||||||||||||||||||
| 2.2.8.1 |
Scope
|
||||||||||||||||||||
| 2.2.8.1.1 |
Corrosive substances are substances which, by chemical action, will cause irreversible damage to the skin, or, in the case of leakage, will materially damage, or even destroy other goods.
|
||||||||||||||||||||
| 2.2.8.1.2 |
Class 8 substances with specific UN numbers shall be classified as stated in the DG List, including the packing groups, subsidiary hazards etc. |
||||||||||||||||||||
|
|
|||||||||||||||||||||
| 2.2.8.1.3 |
For new substances and mixtures which are not specified in the DG List but having properties possible to be classified as Class 8 DG, classification as Class 8 DG and packing group assignments are done on the basis of the length of time of contact necessary to produce irreversible damage to intact skin tissue in accordance with criteria in 2.2.8.2.3 and 2.2.8.2.6 are applied. Alternatively, for mixtures, the criteria in 2.2.8.3 can be used.
|
||||||||||||||||||||
| 2.2.8.2 |
Assignment of packing groups of Class 8 DG
|
||||||||||||||||||||
| 2.2.8.2.1 |
Substances and mixtures of Class 8 are divided among the three packing groups according to their degree of hazard:
|
||||||||||||||||||||
| 2.2.8.2.2 |
Existing human and animal data including information from single or repeated exposure shall be the first line of evaluation, as they give information directly relevant to effects on the skin.
|
||||||||||||||||||||
| 2.2.8.2.3 |
In assigning the packing group, account shall be taken of human experience in instances of accidental exposure. In the absence of human experience, the grouping shall be based on data obtained from experiments in accordance with OECD Test Guidelines24. A substance which is determined not to be corrosive by experiments in accordance with the aforementioned OECD Test Guidelines may be considered not to be corrosive to skin for the purposes of the Code without further testing.
24 OECD Guideline for the testing of chemicals No. 404 Acute Dermal Irritation/ Corrosion 2015. OECD Guideline for testing of chemicals No. 435 in Vitro Membrane Barrier Test Method for Skin Corrosion 2015. OECD Guideline for the testing of chemicals No. 431 in Vitro Skin Corrosion: Reconstructed Human Epidermis (RHE) Test Method 2016. OECD Guideline for the testing of chemicals No. 430 in Vitro Skin Corrosion: Transcutaneous Electrical Resistance Test Method (TER) 2015.
|
||||||||||||||||||||
| 2.2.8.2.4 |
Allocation of substances listed in the DG List to the packing groups in Class 8 has been made on the basis of experience, taking into account such additional factors as inhalation hazard and reactivity with water; a substance or mixture meeting the criteria of Class 8 and having an inhalation toxicity of dusts and mists (LC50) in the range of packing group I, but toxicity through oral ingestion or dermal contact only in the range of packing group III or less, shall be allocated to Class 8 (see 2.1.2.1).
|
||||||||||||||||||||
| 2.2.8.2.5 |
The reactivity of the substance with water (including the formation of dangerous decomposition products) shall also be considered.
|
||||||||||||||||||||
| 2.2.8.2.6 |
Packing groups of Class 8 corrosive substances are assigned in accordance with the following criteria:
|
||||||||||||||||||||
| 2.2.8.2.7 |
Table summarising the criteria in 2.2.8.2.6
|
||||||||||||||||||||
| 2.2.8.3 |
Alternative packing group assignment methods for mixtures: Step-wise approach
|
||||||||||||||||||||
| 2.2.8.3.1 |
General provisions |
||||||||||||||||||||
|
|
|||||||||||||||||||||
| 2.2.8.3.1.1 |
For mixtures it is necessary to obtain or derive information that allows the criteria to be applied to the mixture for the purpose of classification and assignment of packing groups. Depending on the amount of information available for the mixture itself, for similar mixtures and/or for its ingredients, the flow chart of Figure below outlines the process to be followed:
Figure - Step-wise approach to classify and assign packing group of corrosive mixtures
|
||||||||||||||||||||
| 2.2.8.3.2 |
Bridging principles |
||||||||||||||||||||
|
|
|||||||||||||||||||||
| 2.2.8.3.2.1 |
Where a mixture has not been tested to determine its skin corrosion potential, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately classify and assign a packing group for the mixture, these data will be used in accordance with the following bridging principles:
(a) Dilution26: if a tested mixture is diluted with a diluent which does not meet the criteria for Class 8 and does not affect the packing group of other ingredients, then the new diluted mixture may be assigned to the same packing group as the original tested mixture.
(b) Batching: the skin corrosion potential of a tested production batch of a mixture can be assumed to be substantially equivalent to that of another untested production batch of the same commercial product when produced by or under the control of the same manufacturer, unless there is reason to believe there is significant variation such that the skin corrosion potential of the untested batch has changed. If the latter occurs, a new classification is necessary.
(c) Concentration of mixtures of packing group I: if a tested mixture meeting the criteria for inclusion in packing group I is concentrated, the more concentrated untested mixture may be assigned to packing group I without additional testing.
(d) Interpolation within one packing group: for three mixtures (A, B and C) with identical ingredients, where mixtures A and B have been tested and are in the same skin corrosion packing group, and where untested mixture C has the same Class 8 ingredients as mixtures A and B but has concentrations of Class 8 ingredients intermediate to the concentrations in mixtures A and B, then mixture C is assumed to be in the same skin corrosion packing group as A and B.
(e) Substantially similar mixtures: given the following:
(i) Two mixtures: (A+B) and (C+B); (ii) The concentration of ingredient B is the same in both mixtures; (iii) The concentration of ingredient A in mixture (A+B) equals the concentration of ingredient C in mixture (C+B); (iv) Data on skin corrosion for ingredients A and C are available and substantially equivalent, i.e. they are the same skin corrosion packing group and do not affect the skin corrosion potential of B. If mixture (A+B) or (C+B) is already classified based on test data, then the other mixture may be assigned to the same packing group. |
||||||||||||||||||||
|
|
|||||||||||||||||||||
| 2.2.8.3.3 |
Calculation method based on the classification of the substances |
||||||||||||||||||||
|
|
|||||||||||||||||||||
| 2.2.8.3.3.1 |
Where a mixture has not been tested to determine its skin corrosion potential, nor is sufficient data available on similar mixtures, the corrosive properties of the substances in the mixture shall be considered to classify and assign a packing group. |
||||||||||||||||||||
|
|
|||||||||||||||||||||
| 2.2.8.3.3.2 |
The calculation method stated in 2.8.4.3 in IMDG Code or 2.8.4.3 in Recommendations on the Transport of Dangerous Goods Model Regulations shall be applied. It should be noted that applying the calculation method is only allowed if there are no synergistic effects that make the mixture more corrosive than the sum of its substances. This restriction applies only if packing group II or III would be assigned to the mixture. |
||||||||||||||||||||
|
|
|||||||||||||||||||||
| 2.2.8.3.3.3 |
When using the calculation method, all Class 8 ingredients present at a concentration of ≥ 1% shall be taken into account, or < 1% if these ingredients are still relevant for classifying the mixture to be corrosive to skin. |
| 2.2.9 |
Class 9 - Miscellaneous Dangerous Substances or Materials
|
| 2.2.9.1 |
Scope
|
| 2.2.9.1.1 |
For the purposes of the Ordinance, Class 9 DG are substances which present a danger not covered by other classes. Class 9 DG are listed in Part 2 of Schedule 2 of Cap. 295E which includes DG in the following table.
|
| UN No | Proper Shipping Name | Packing Group | |
| 1841 |
ACETALDEHYDE AMMONIA |
III | |
| 1941 |
DIBROMODIFLUOROMETHANE |
III | |
| 1990 | BENZALDEHYDE | III | |
| 2211 | POLYMERIC BEADS, EXPANDABLE, evolving flammable vapour | III |